Practical therapeutic drug management in HIV-infected patients: use of population pharmacokinetic models supplemented by individualized Bayesian dose optimization
- PMID: 18635757
- PMCID: PMC2724306
- DOI: 10.1177/0091270008321789
Practical therapeutic drug management in HIV-infected patients: use of population pharmacokinetic models supplemented by individualized Bayesian dose optimization
Abstract
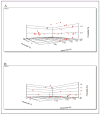
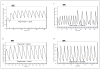
Individualized, model-based, target-oriented optimal concentration-controlled dosing of HIV medications can be beneficial to patients for whom there are limited dosing guidelines, such as children, adolescents, or patients with altered physiologic function. Barriers to this approach include lack of training, expertise, and access to appropriate software to assist the clinician. The authors present 4 illustrative clinical cases of HIV-infected patients whose therapy was optimized using population pharmacokinetic models (here generated from published studies) and supplemented by individualized Bayesian adaptive control of dosage regimens as implemented in the MM-USCPACK software. These 4 cases illustrate how clinicians can maximize therapeutic success in (1) patients with reduced drug clearance, (2) young adolescents transitioning to adult physiology, (3) patients with dose-dependent toxicity, and (4) adolescents with limited therapeutic options.
Figures

References
-
- Fraaij PL, van Kampen JJ, Burger DM, de Groot R. Pharmacokinetics of antiretroviral therapy in HIV-1-infected children. Clin Pharmacokinet. 2005;44(9):935–956. - PubMed
-
- Reed MD, Besunder JB. Developmental pharmacology: ontogenic basis of drug disposition. Pediatr Clin North Am. 1989;36(5):1053–1074. - PubMed
-
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. - PubMed
-
- Lent-Evers NA, Mathot RA, Geus WP, van Hout BA, Vinks AA. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit. 1999;21(1):63–73. - PubMed
-
- Bleyzac N, Souillet G, Magron P, et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001;28(8):743–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

