Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment
- PMID: 18635807
- PMCID: PMC2582147
- DOI: 10.1126/science.1157632
Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment
Abstract
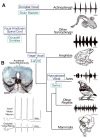
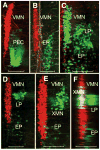
The macroevolutionary events leading to neural innovations for social communication, such as vocalization, are essentially unexplored. Many fish vocalize during female courtship and territorial defense, as do amphibians, birds, and mammals. Here, we map the neural circuitry for vocalization in larval fish and show that the vocal network develops in a segment-like region across the most caudal hindbrain and rostral spinal cord. Taxonomic analysis demonstrates a highly conserved pattern between fish and all major lineages of vocal tetrapods. We propose that the vocal basis for acoustic communication among vertebrates evolved from an ancestrally shared developmental compartment already present in the early fishes.
Figures
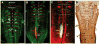

Comment in
-
Neuroscience. Vertebrate vocalizations.Science. 2008 Jul 18;321(5887):347-8. doi: 10.1126/science.1161775. Science. 2008. PMID: 18635781 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

