The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival
- PMID: 18636077
- PMCID: PMC2742618
- DOI: 10.1038/cdd.2008.109
The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival
Abstract
Mdm2 is the major inhibitor of the p53 tumor suppressor. Loss of Mdm2 in mice or in specific tissues of the mouse always yields p53-dependent lethal phenotypes. However, the role of Mdm2 in tissues with high turnover capacity is unknown. We have engineered mice lacking Mdm2 in the intestinal epithelium using the Cre/LoxP system. Loss of Mdm2 (Mdm2(intDelta)) results in viable animals, but neonates display multiple intestinal abnormalities such as hyperplasia, enterocyte vacuolization, and inflammation. These defects correlate with a drastic increase in p53-dependent apoptosis in highly proliferative and differentiated cells. Unexpectedly, the observed phenotypes disappear with age. The tissue selects against Mdm2-null cells and increases its proliferative capacity. Additionally, the intestinal stem and progenitor cell populations are enriched leading to an increase in crypt fission events. Enhanced proliferation is achieved by activation of the canonical Wnt and EGFR-mediated Ras/MAPK pathways. While Mdm2 is a critical inhibitor of p53 in the intestinal epithelium, the tissue employs a series of processes that compensate for cell death.
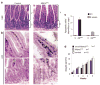
Figures





References
-
- Vousden KH. p53: death star. Cell. 2000;103:691–694. - PubMed
-
- Evans SC, Viswanathan M, Grier JD, Narayana M, El-Naggar AK, Lozano G. An alternatively spliced HDM2 product increases p53 activity by inhibiting HDM2. Oncogene. 2001;20:4041–4049. - PubMed
-
- Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

