Emergent synchronous bursting of oxytocin neuronal network
- PMID: 18636098
- PMCID: PMC2440533
- DOI: 10.1371/journal.pcbi.1000123
Emergent synchronous bursting of oxytocin neuronal network
Abstract
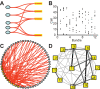
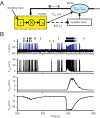
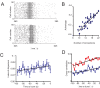
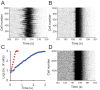
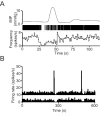
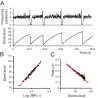
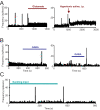
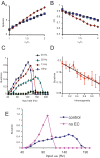
When young suckle, they are rewarded intermittently with a let-down of milk that results from reflex secretion of the hormone oxytocin; without oxytocin, newly born young will die unless they are fostered. Oxytocin is made by magnocellular hypothalamic neurons, and is secreted from their nerve endings in the pituitary in response to action potentials (spikes) that are generated in the cell bodies and which are propagated down their axons to the nerve endings. Normally, oxytocin cells discharge asynchronously at 1-3 spikes/s, but during suckling, every 5 min or so, each discharges a brief, intense burst of spikes that release a pulse of oxytocin into the circulation. This reflex was the first, and is perhaps the best, example of a physiological role for peptide-mediated communication within the brain: it is coordinated by the release of oxytocin from the dendrites of oxytocin cells; it can be facilitated by injection of tiny amounts of oxytocin into the hypothalamus, and it can be blocked by injection of tiny amounts of oxytocin antagonist. Here we show how synchronized bursting can arise in a neuronal network model that incorporates basic observations of the physiology of oxytocin cells. In our model, bursting is an emergent behaviour of a complex system, involving both positive and negative feedbacks, between many sparsely connected cells. The oxytocin cells are regulated by independent afferent inputs, but they interact by local release of oxytocin and endocannabinoids. Oxytocin released from the dendrites of these cells has a positive-feedback effect, while endocannabinoids have an inhibitory effect by suppressing the afferent input to the cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol. 1999;57:625–655. - PubMed
-
- Hatton GI. Astroglial modulation of neurotransmitter/peptide release from the neurohypophysis: present status. J Chem Neuroanat. 1999;16:203–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

