Protein kinase C isoenzymes differentially regulate the differentiation-dependent expression of adhesion molecules in human epidermal keratinocytes
- PMID: 18637128
- PMCID: PMC6957249
- DOI: 10.1111/j.1600-0625.2008.00771.x
Protein kinase C isoenzymes differentially regulate the differentiation-dependent expression of adhesion molecules in human epidermal keratinocytes
Abstract
Epidermal expression of adhesion molecules such as desmogleins (Dsg) and cadherins is strongly affected by the differentiation status of keratinocytes. We have previously shown that certain protein kinase C (PKC) isoforms differentially alter the growth and differentiation of human epidermal HaCaT keratinocytes. In this paper, using recombinant overexpression and RNA interference, we define the specific roles of the different PKC isoenzymes in modulation of expression of adhesion molecules in HaCaT keratinocytes. The level of Dsg1, a marker of differentiating keratinocytes, was antagonistically regulated by two Ca-independent 'novel' nPKC isoforms; i.e. it increased by the differentiation-promoting nPKCdelta and decreased by the growth-promoting nPKCepsilon. The expression of Dsg3 (highly expressed in proliferating epidermal layers) was conversely regulated by these isoenzymes, and was also inhibited by the differentiation inducer Ca-dependent 'conventional' cPKCalpha. Finally, the expression of P-cadherin (a marker of proliferating keratinocytes) was regulated by all of the examined PKCs, also in an antagonistic manner (inhibited by cPKCalpha/nPKCdelta and stimulated by cPKCbeta/nPKCepsilon). Collectively, the presented results strongly argue for the marked, differential, and in some instances antagonistic roles of individual Ca-dependent and Ca-independent PKC isoforms in the regulation of expression of adhesion molecules of desmosomes and adherent junctions in human epidermal keratinocytes.
Figures
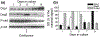
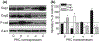

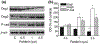
References
-
- Koch PJ, Franke WW. Desmosomal cadherins: another growing multigene family of adhesin molecules. Curr Opin Cell Biol 1994: 6: 682–687. - PubMed
-
- North AJ, Bardsley WG, Hyam J et al. Molecular map of the desmosomal plague. J Cell Sci 1999: 112: 4325–4336. - PubMed
-
- Amagai M, Ahmed AR, Kitajima Y et al. Are desmoglein auto-antibodies essential for the immunopathogenesis of pemphigus vulgaris, or just ‘witnesses of disease’? Exp Dermatol 2006: 15: 815–831. - PubMed
-
- Garrod DR, Merritt AJ, Nie Z. Desmosomal cadherins. Curr Opin Cell Biol 2002: 14: 537–545. - PubMed
-
- Ishikawa H, Li K, Tamai K, Sawamura D, Uitto J. Cloning of the mouse desmoglein 3 gene (Dsg3): interspecies conservation within the cadherin superfamily. Exp Dermatol 2000: 9: 229–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

