Profiling of serum and tissue high abundance acute-phase proteins of patients with epithelial and germ line ovarian carcinoma
- PMID: 18637207
- PMCID: PMC2496906
- DOI: 10.1186/1477-5956-6-20
Profiling of serum and tissue high abundance acute-phase proteins of patients with epithelial and germ line ovarian carcinoma
Abstract
Background: Acute-phase response involves the simultaneous altered expression of serum proteins in association to inflammation, infection, injury or malignancy. Studies of the acute-phase response usually involve determination of the levels of individual acute-phase serum proteins. In the present study, the acute-phase response of patients with epithelial (EOCa) and germ-line (GOCa) ovarian carcinoma was investigated using the gel-based proteomic approach, a technique which allowed the simultaneous assessment of the levels of the acute-phase serum high abundance proteins. Data obtained were validated using ELISA and immunostaining of biopsy samples.
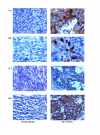
Results: Enhanced expression of clusterin (CLU), alpha1-antitrypsin, haptoglobin and leucine rich glycoprotein was detected in all patients. However, the levels of alpha1-antichymotrypsin (ACT) was only enhanced in EOCa patients, while patients with GOCa were typically characterized by elevated levels of ceruloplasmin but lower levels of alpha2-HS glycoprotein. The enhanced expression of CLU in EOCa and GOCa patients and up-regulated expression of ACT specifically in EOCa patients were confirmed by ELISA. Immunohistochemical staining of biopsy samples of EOCa and GOCa patients demonstrated correlation of the acute-phase protein expression.
Conclusion: Patients with EOCa and GOCa demonstrated distinctive aberrant expression of serum and tissue high abundance acute-phase proteins compared to negative control women.
Figures



References
-
- Pieper R, Gatlin CL, Makusky AJ, Russo PS, Schatz CR, Miller SS, Su Q, McGrath AM, Estock MA, Parmar PP, Zhao M, Huang ST, Zhou J, Wang F, Esquer-Blasco R, Anderson NL, Taylor J, Steiner S. The human serum proteome: Display of nearly 3700 chromatographically separated protein spots on two-dimensional electrophoresis gels and identification of 325 distinct proteins. Proteomics. 2003;3:1345–1364. doi: 10.1002/pmic.200300449. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

