Cell wounding and repair in ventilator injured lungs
- PMID: 18638574
- PMCID: PMC2651843
- DOI: 10.1016/j.resp.2008.06.019
Cell wounding and repair in ventilator injured lungs
Abstract
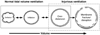
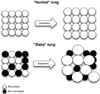
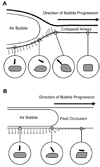
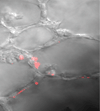
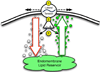
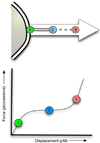
Acute lung injury (ALI) is a common, frequently hospital-acquired condition with a high morbidity and mortality. The stress associated with invasive mechanical ventilation represents a potentially harmful exposure, and attempts to minimize deforming stress through low tidal ventilation have proven efficacious. Lung cells are both sensors and transducers of deforming stress, and are frequently wounded in the setting of mechanical ventilation. Cell wounding may be one of the drivers of the innate immunologic and systemic inflammatory response associated with mechanical ventilation. These downstream effects of mechanotransduction have been referred to collectively as "Biotrauma". Our review will focus on cellular stress failure, that is cell wounding, and the mechanisms mediating subsequent plasma membrane repair, we hold that a better mechanistic understanding of cell plasticity, deformation associated remodeling and repair will reveal candidate approaches for lung protective interventions in mechanically ventilated patients. We will detail one such intervention, lung conditioning with hypertonic solutions as an example of ongoing research in this arena.
Figures

References
-
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. - PubMed
-
- Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–S199. - PubMed
-
- Bachofen H, Schurch S. Alveolar surface forces and lung architecture. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:183–193. - PubMed
-
- Bilek AM, Dee KC, Gaver DP., 3rd Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol. 2003;94:770–783. - PubMed
-
- Caruso P, Meireles SI, Reis LF, Mauad T, Martins MA, Deheinzelin D. Low tidal volume ventilation induces proinflammatory and profibrogenic response in lungs of rats. Intensive Care Med. 2003;29:1808–1811. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

