Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators
- PMID: 18640274
- PMCID: PMC2577133
- DOI: 10.1016/j.ceb.2008.06.008
Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators
Abstract
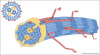
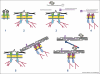
Collagens are triple helical proteins that occur in the extracellular matrix (ECM) and at the cell-ECM interface. There are more than 30 collagens and collagen-related proteins but the most abundant are collagens I and II that exist as D-periodic (where D = 67 nm) fibrils. The fibrils are of broad biomedical importance and have central roles in embryogenesis, arthritis, tissue repair, fibrosis, tumor invasion, and cardiovascular disease. Collagens I and II spontaneously form fibrils in vitro, which shows that collagen fibrillogenesis is a selfassembly process. However, the situation in vivo is not that simple; collagen I-containing fibrils do not form in the absence of fibronectin, fibronectin-binding and collagen-binding integrins, and collagen V. Likewise, the thin collagen II-containing fibrils in cartilage do not form in the absence of collagen XI. Thus, in vivo, cellular mechanisms are in place to control what is otherwise a protein self-assembly process. This review puts forward a working hypothesis for how fibronectin and integrins (the organizers) determine the site of fibril assembly, and collagens V and XI (the nucleators) initiate collagen fibrillogenesis.
Figures
References
-
- Gross J., Kirk D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958;233:355–360. - PubMed
-
- Prockop D.J., Fertala A. Inhibition of the self-assembly of collagen I into fibrils with synthetic peptides. Demonstration that assembly is driven by specific binding sites on the monomers. J Biol Chem. 1998;273:15598–15604. - PubMed
-
- Greenspan D.S. Biosynthetic processing of collagen molecules. Top Curr Chem. 2005;247:149–183.
-
- Kadler K.E., Hojima Y., Prockop D.J. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem. 1987;262:15696–15701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

