Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology
- PMID: 18640912
- PMCID: PMC2607335
- DOI: 10.1098/rstb.2008.0107
Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology
Abstract
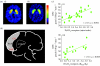
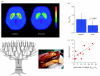
Drugs and food exert their reinforcing effects in part by increasing dopamine (DA) in limbic regions, which has generated interest in understanding how drug abuse/addiction relates to obesity. Here, we integrate findings from positron emission tomography imaging studies on DA's role in drug abuse/addiction and in obesity and propose a common model for these two conditions. Both in abuse/addiction and in obesity, there is an enhanced value of one type of reinforcer (drugs and food, respectively) at the expense of other reinforcers, which is a consequence of conditioned learning and resetting of reward thresholds secondary to repeated stimulation by drugs (abuse/addiction) and by large quantities of palatable food (obesity) in vulnerable individuals (i.e. genetic factors). In this model, during exposure to the reinforcer or to conditioned cues, the expected reward (processed by memory circuits) overactivates the reward and motivation circuits while inhibiting the cognitive control circuit, resulting in an inability to inhibit the drive to consume the drug or food despite attempts to do so. These neuronal circuits, which are modulated by DA, interact with one another so that disruption in one circuit can be buffered by another, which highlights the need of multiprong approaches in the treatment of addiction and obesity.
Figures



References
-
- Allison D.B, Mentore J.L, Heo M, Chandler L.P, Cappelleri J.C, Infante M.C, Weiden P.J. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am. J. Psychiatry. 1999;156:1686–1696. - PubMed
-
- Avena N.M, Rada P, Hoebel B.G. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2008;32:20–39. doi:10.1016/j.neubiorev.2007.04.019 - DOI - PMC - PubMed
-
- Berthoud H.R. Interactions between the ‘cognitive’ and ‘metabolic’ brain in the control of food intake. Physiol. Behav. 2007;91:486–498. doi:10.1016/j.physbeh.2006.12.016 - DOI - PubMed
-
- Garavan H, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry. 2000;157:1789–1798. doi:10.1176/appi.ajp.157.11.1789 - DOI - PubMed
-
- Gautier J.F, Chen K, Salbe A.D, Bandy D, Pratley R.E, Heiman M, Ravussin E, Reiman E.M, Tataranni P.A. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49:838–846. doi:10.2337/diabetes.49.5.838 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
