Review. Neurobiology of nicotine dependence
- PMID: 18640919
- PMCID: PMC2607327
- DOI: 10.1098/rstb.2008.0095
Review. Neurobiology of nicotine dependence
Abstract
Nicotine is a psychoactive ingredient in tobacco that significantly contributes to the harmful tobacco smoking habit. Nicotine dependence is more prevalent than dependence on any other substance. Preclinical research in animal models of the various aspects of nicotine dependence suggests a critical role of glutamate, gamma-aminobutyric acid (GABA), cholinergic and dopamine neurotransmitter interactions in the ventral tegmental area and possibly other brain sites, such as the central nucleus of the amygdala and the prefrontal cortex, in the effects of nicotine. Specifically, decreasing glutamate transmission or increasing GABA transmission with pharmacological manipulations decreased the rewarding effects of nicotine and cue-induced reinstatement of nicotine seeking. Furthermore, early nicotine withdrawal is characterized by decreased function of presynaptic inhibitory metabotropic glutamate 2/3 receptors and increased expression of postsynaptic glutamate receptor subunits in limbic and frontal brain sites, while protracted abstinence may be associated with increased glutamate response to stimuli associated with nicotine administration. Finally, adaptations in nicotinic acetylcholine receptor function are also involved in nicotine dependence. These neuroadaptations probably develop to counteract the decreased glutamate and cholinergic transmission that is hypothesized to characterize early nicotine withdrawal. In conclusion, glutamate, GABA and cholinergic transmission in limbic and frontal brain sites are critically involved in nicotine dependence.
Figures


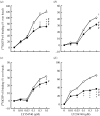
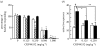
References
-
- Anthony J.C, Warner L.A, Kessler R.C. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp. Clin. Psychopharmacol. 1994;2:244–268. doi:10.1037/1064-1297.2.3.244 - DOI
-
- Bardo M.T, Green T.A, Crooks P.A, Dwoskin L.P. Nornicotine is self-administered intravenously by rats. Psychopharmacologia. 1999;146:290–296. doi:10.1007/s002130051119 - DOI - PubMed
-
- Belluzzi J.D, Wang R, Leslie F.M. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropschopharmacology. 2005;30:705–712. doi:10.1038/sj.npp.1300586 - DOI - PubMed
-
- Bespalov A.Y, Dravolina O.A, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49(Suppl. 1):167–178. doi:10.1016/j.neuropharm.2005.06.007 - DOI - PubMed
-
- Besson M, et al. Long-term effects of chronic nicotine exposure on brain nicotinic receptors. Proc. Natl Acad. Sci. USA. 2007;104:8155–8160. doi:10.1073/pnas.0702698104 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
