Caenorhabditis elegans gelsolin-like protein 1 is a novel actin filament-severing protein with four gelsolin-like repeats
- PMID: 18640981
- PMCID: PMC2533794
- DOI: 10.1074/jbc.M803618200
Caenorhabditis elegans gelsolin-like protein 1 is a novel actin filament-severing protein with four gelsolin-like repeats
Abstract
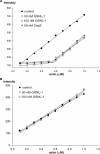
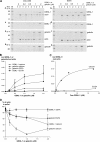
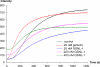
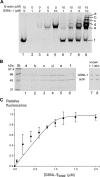
The gelsolin family of proteins is a major class of actin regulatory proteins that sever, cap, and nucleate actin filaments in a calcium-dependent manner and are involved in various cellular processes. Typically, gelsolin-related proteins have three or six repeats of gelsolin-like (G) domain, and each domain plays a distinct role in severing, capping, and nucleation. The Caenorhabditis elegans gelsolin-like protein-1 (gsnl-1) gene encodes an unconventional gelsolin-related protein with four G domains. Sequence alignment suggests that GSNL-1 lacks two G domains that are equivalent to fourth and fifth G domains of gelsolin. In vitro, GSNL-1 severed actin filaments and capped the barbed end in a calcium-dependent manner. However, unlike gelsolin, GSNL-1 remained bound to the side of F-actin with a submicromolar affinity and did not nucleate actin polymerization, although it bound to G-actin with high affinity. These results indicate that GSNL-1 is a novel member of the gelsolin family of actin regulatory proteins and provide new insight into functional diversity and evolution of gelsolin-related proteins.
Figures




Similar articles
-
Distinct roles of four gelsolin-like domains of Caenorhabditis elegans gelsolin-like protein-1 in actin filament severing, barbed end capping, and phosphoinositide binding.Biochemistry. 2010 May 25;49(20):4349-60. doi: 10.1021/bi100215b. Biochemistry. 2010. PMID: 20392036 Free PMC article.
-
Regulatory role of the second gelsolin-like domain of Caenorhabditis elegans gelsolin-like protein 1 (GSNL-1) in its calcium-dependent conformation and actin-regulatory activities.Cytoskeleton (Hoboken). 2013 Apr;70(4):228-39. doi: 10.1002/cm.21103. Epub 2013 Mar 21. Cytoskeleton (Hoboken). 2013. PMID: 23475707 Free PMC article.
-
Calcium-sensitive activity and conformation of Caenorhabditis elegans gelsolin-like protein 1 are altered by mutations in the first gelsolin-like domain.J Biol Chem. 2011 Sep 30;286(39):34051-9. doi: 10.1074/jbc.M111.237404. Epub 2011 Aug 12. J Biol Chem. 2011. PMID: 21840993 Free PMC article.
-
Arabidopsis VILLIN1 generates actin filament cables that are resistant to depolymerization.Plant Cell. 2005 Feb;17(2):486-501. doi: 10.1105/tpc.104.028555. Epub 2005 Jan 19. Plant Cell. 2005. PMID: 15659626 Free PMC article.
-
The gelsolin family of actin regulatory proteins: modular structures, versatile functions.FEBS Lett. 2003 Sep 25;552(2-3):75-81. doi: 10.1016/s0014-5793(03)00932-3. FEBS Lett. 2003. PMID: 14527663 Review.
Cited by
-
TranSeqAnnotator: large-scale analysis of transcriptomic data.BMC Bioinformatics. 2012;13 Suppl 17(Suppl 17):S24. doi: 10.1186/1471-2105-13-S17-S24. Epub 2012 Dec 13. BMC Bioinformatics. 2012. PMID: 23282024 Free PMC article.
-
The Cell Death Pathway Regulates Synapse Elimination through Cleavage of Gelsolin in Caenorhabditis elegans Neurons.Cell Rep. 2015 Jun 23;11(11):1737-48. doi: 10.1016/j.celrep.2015.05.031. Epub 2015 Jun 11. Cell Rep. 2015. PMID: 26074078 Free PMC article.
-
Dynamic regulation of sarcomeric actin filaments in striated muscle.Cytoskeleton (Hoboken). 2010 Nov;67(11):677-92. doi: 10.1002/cm.20476. Cytoskeleton (Hoboken). 2010. PMID: 20737540 Free PMC article. Review.
-
Transcriptomic and Proteomic Analysis of Marine Nematode Litoditis marina Acclimated to Different Salinities.Genes (Basel). 2022 Apr 7;13(4):651. doi: 10.3390/genes13040651. Genes (Basel). 2022. PMID: 35456458 Free PMC article.
-
The Caenorhabditis elegans TDRD5/7-like protein, LOTR-1, interacts with the helicase ZNFX-1 to balance epigenetic signals in the germline.PLoS Genet. 2022 Jun 3;18(6):e1010245. doi: 10.1371/journal.pgen.1010245. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35657999 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

