Structure basis and unconventional lipid membrane binding properties of the PH-C1 tandem of rho kinases
- PMID: 18640982
- PMCID: PMC3258851
- DOI: 10.1074/jbc.M803417200
Structure basis and unconventional lipid membrane binding properties of the PH-C1 tandem of rho kinases
Abstract
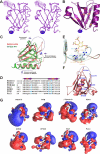
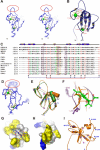
Rho kinase (ROCK), a downstream effector of Rho GTPase, is a serine/threonine protein kinase that regulates many crucial cellular processes via control of cytoskeletal structures. The C-terminal PH-C1 tandem of ROCKs has been implicated to play an autoinhibitory role by sequestering the N-terminal kinase domain and reducing its kinase activity. The binding of lipids to the pleckstrin homology (PH) domain not only regulates the localization of the protein but also releases the kinase domain from the close conformation and thereby activates its kinase activity. However, the molecular mechanism governing the ROCK PH-C1 tandem-mediated lipid membrane interaction is not known. In this study, we demonstrate that ROCK is a new member of the split PH domain family of proteins. The ROCK split PH domain folds into a canonical PH domain structure. The insertion of the atypical C1 domain in the middle does not alter the structure of the PH domain. We further show that the C1 domain of ROCK lacks the diacylglycerol/phorbol ester binding pocket seen in other canonical C1 domains. Instead, the inserted C1 domain and the PH domain function cooperatively in binding to membrane bilayers via the unconventional positively charged surfaces on each domain. Finally, the analysis of all split PH domains with known structures indicates that split PH domains represent a unique class of tandem protein modules, each possessing distinct structural and functional features.
Figures



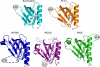
References
-
- Wen, W., Yan, J., and Zhang, M. (2006) J. Biol. Chem. 281 12060-12068 - PubMed
-
- Teo, H., Gill, D. J., Sun, J., Perisic, O., Veprintsev, D. B., Vallis, Y., Emr, S. D., and Williams, R. L. (2006) Cell 125 99-111 - PubMed
-
- Yan, J., Wen, W., Chan, L. N., and Zhang, M. (2008) J. Mol. Biol. 378 425-435 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

