Protein phosphatase 1 regulates the phosphorylation state of the polarity scaffold Par-3
- PMID: 18641122
- PMCID: PMC2475498
- DOI: 10.1073/pnas.0804102105
Protein phosphatase 1 regulates the phosphorylation state of the polarity scaffold Par-3
Abstract
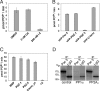
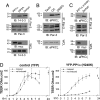
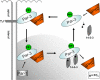
Phosphorylation of the polarity protein Par-3 by the serine/threonine kinases aPKCzeta/iota and Par-1 (EMK1/MARK2) regulates various aspects of epithelial cell polarity, but little is known about the mechanisms by which these posttranslational modifications are reversed. We find that the serine/threonine protein phosphatase PP1 (predominantly the alpha isoform) binds Par-3, which localizes to tight junctions in MDCKII cells. PP1alpha can associate with multiple sites on Par-3 while retaining its phosphatase activity. By using a quantitative mass spectrometry-based technique, multiple reaction monitoring, we show that PP1alpha specifically dephosphorylates Ser-144 and Ser-824 of mouse Par-3, as well as a peptide encompassing Ser-885. Consistent with these observations, PP1alpha regulates the binding of 14-3-3 proteins and the atypical protein kinase C (aPKC) zeta to Par-3. Furthermore, the induced expression of a catalytically inactive mutant of PP1alpha severely delays the formation of functional tight junctions in MDCKII cells. Collectively, these results show that Par-3 functions as a scaffold, coordinating both serine/threonine kinases and the PP1alpha phosphatase, thereby providing dynamic control of the phosphorylation events that regulate the Par-3/aPKC complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kemphues K. PARsing embryonic polarity. Cell. 2000;101:345–348. - PubMed
-
- Ohno S. Intercellular junctions and cellular polarity: The PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13:641–648. - PubMed
-
- Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. - PubMed
-
- Lin D, et al. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–547. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

