Prevention of aortic fibrosis by N-acetyl-seryl-aspartyl-lysyl-proline in angiotensin II-induced hypertension
- PMID: 18641275
- PMCID: PMC2544498
- DOI: 10.1152/ajpheart.00481.2008
Prevention of aortic fibrosis by N-acetyl-seryl-aspartyl-lysyl-proline in angiotensin II-induced hypertension
Abstract
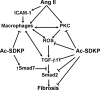
Fibrosis is an important component of large conduit artery disease in hypertension. The endogenous tetrapeptide N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) has anti-inflammatory and antifibrotic effects in the heart and kidney. However, it is not known whether Ac-SDKP has an anti-inflammatory and antifibrotic effect on conduit arteries such as the aorta. We hypothesize that in ANG II-induced hypertension Ac-SDKP prevents aortic fibrosis and that this effect is associated with decreased protein kinase C (PKC) activation, leading to reduced oxidative stress and inflammation and a decrease in the profibrotic cytokine transforming growth factor-beta1 (TGF-beta1) and phosphorylation of its second messenger Smad2. To test this hypothesis we used rats with ANG II-induced hypertension and treated them with either vehicle or Ac-SDKP. In this hypertensive model we found an increased collagen deposition and collagen type I and III mRNA expression in the aorta. These changes were associated with increased PKC activation, oxidative stress, intercellular adhesion molecule (ICAM)-1 mRNA expression, and macrophage infiltration. TGF-beta1 expression and Smad2 phosphorylation also increased. Ac-SDKP prevented these effects without decreasing blood pressure or aortic hypertrophy. Ac-SDKP also enhanced expression of inhibitory Smad7. These data indicate that in ANG II-induced hypertension Ac-SDKP has an aortic antifibrotic effect. This effect may be due in part to inhibition of PKC activation, which in turn could reduce oxidative stress, ICAM-1 expression, and macrophage infiltration. Part of the effect of Ac-SDKP could also be due to reduced expression of the profibrotic cytokine TGF-beta1 and inhibition of Smad2 phosphorylation.
Figures


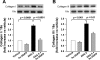





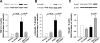
Similar articles
-
Antifibrotic effect of Ac-SDKP and angiotensin-converting enzyme inhibition in hypertension.J Hypertens. 2004 Mar;22(3):593-603. doi: 10.1097/00004872-200403000-00023. J Hypertens. 2004. PMID: 15076166 Free PMC article.
-
Antifibrotic effects of N-acetyl-seryl-aspartyl-Lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats.Hypertension. 2001 Feb;37(2 Pt 2):794-800. doi: 10.1161/01.hyp.37.2.794. Hypertension. 2001. PMID: 11230375 Free PMC article.
-
Prevention of myocardial fibrosis by N-acetyl-seryl-aspartyl-lysyl-proline in diabetic rats.Clin Sci (Lond). 2009 Oct 26;118(3):211-20. doi: 10.1042/cs20090234. Clin Sci (Lond). 2009. PMID: 20310083
-
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP): Potential target molecule in research of heart, kidney and brain.Curr Pharm Des. 2015;21(35):5135-43. doi: 10.2174/1381612821666150909093927. Curr Pharm Des. 2015. PMID: 26350537 Review.
-
The anti-inflammatory peptide Ac-SDKP: Synthesis, role in ACE inhibition, and its therapeutic potential in hypertension and cardiovascular diseases.Pharmacol Res. 2018 Aug;134:268-279. doi: 10.1016/j.phrs.2018.07.006. Epub 2018 Jul 7. Pharmacol Res. 2018. PMID: 29990624 Review.
Cited by
-
Rediscovering ACE: novel insights into the many roles of the angiotensin-converting enzyme.J Mol Med (Berl). 2013 Oct;91(10):1143-54. doi: 10.1007/s00109-013-1051-z. Epub 2013 May 18. J Mol Med (Berl). 2013. PMID: 23686164 Free PMC article. Review.
-
The Role of Tβ4-POP-Ac-SDKP Axis in Organ Fibrosis.Int J Mol Sci. 2022 Oct 31;23(21):13282. doi: 10.3390/ijms232113282. Int J Mol Sci. 2022. PMID: 36362069 Free PMC article. Review.
-
Fragment-based design for the development of N-domain-selective angiotensin-1-converting enzyme inhibitors.Clin Sci (Lond). 2014 Feb;126(4):305-13. doi: 10.1042/CS20130403. Clin Sci (Lond). 2014. PMID: 24015848 Free PMC article.
-
A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme.Pharmacol Rev. 2012 Dec 20;65(1):1-46. doi: 10.1124/pr.112.006809. Print 2013 Jan. Pharmacol Rev. 2012. PMID: 23257181 Free PMC article. Review.
-
N-acetyl-seryl-aspartyl-lysyl-proline attenuates renal injury and dysfunction in hypertensive rats with reduced renal mass: council for high blood pressure research.Hypertension. 2010 Feb;55(2):459-67. doi: 10.1161/HYPERTENSIONAHA.109.144568. Epub 2009 Dec 21. Hypertension. 2010. PMID: 20026760 Free PMC article.
References
-
- Alexander RW Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension 25: 155–161, 1995. - PubMed
-
- Azizi M, Ezan E, Nicolet L, Grognet JM, Menard J. High plasma level of N-acetyl-seryl-aspartyl-lysyl-proline: a new marker of chronic angiotensin-converting enzyme inhibition. Hypertension 30: 1015–1019, 1997. - PubMed
-
- Benetos A, Levy BI, Lacolley P, Taillard F, Duriez M, Safar ME. Role of angiotensin II and bradykinin on aortic collagen following converting enzyme inhibition in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 17: 3196–3201, 1997. - PubMed
-
- Bezie Y, Lamaziere JM, Laurent S, Challande P, Cunha RS, Bonnet J, Lacolley P. Fibronectin expression and aortic wall elastic modulus in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 18: 1027–1034, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

