Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling
- PMID: 18641276
- PMCID: PMC2544502
- DOI: 10.1152/ajpheart.00400.2008
Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling
Abstract
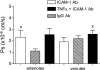
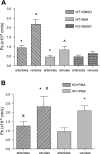
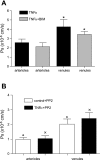
Two key characteristics of the inflammatory response are the recruitment of leukocytes to inflamed tissue as well as changes in vessel permeability. We explored the relationship between these two processes using intravital confocal microscopy in cremasters of anesthetized (65 mg/kg Nembutal ip) mice. We provide direct evidence that intercellular adhesion molecule-1 (ICAM-1) links leukocyte-endothelial cell interactions and changes in solute permeability (Ps). Importantly, we show that arterioles, not just venules, respond to proinflammatory stimuli, thus contributing to microvascular exchange. We identified two independent, ICAM-1-mediated pathways regulating Ps. Under control conditions in wild-type (WT) mice, there is a constitutive PKC-dependent pathway (Ps = 1.0 +/- 0.10 and 2.2 +/- 0.46 x 10(-6) cm/s in arterioles and venules, respectively), which was significantly reduced in ICAM-1 knockout (KO) mice (Ps = 0.54 +/- 0.07 and 0.77 +/- 0.11 x 10(-6) cm/s). The PKC inhibitor bisindolylmaleimid l (1 micromol/l in 0.01% DMSO) decreased P(s) in WT mice to levels similar to those in ICAM-1 KO mice. Likewise, a PKC activator (phorbol-12-myristate-acetate; 1 micromol/l in 0.01% DMSO) successfully restored Ps in ICAM-1 KO vessels to be not different from that of the WT controls. On the other hand, during TNF-alpha-induced inflammation, Ps in WT mice was significantly increased (2-fold in venules and 2.5-fold in arterioles) in a Src-dependent and PKC-independent manner. The blockade of Src (PP2; 2 micromol/l in 0.01% DMSO) but not PKC significantly reduced the TNF-alpha-dependent increase in Ps. We conclude that ICAM-1 plays an essential role in the regulation of Ps in microvessels and that there are two separate (constitutive and inducible) signaling pathways that regulate permeability under normal and inflamed conditions.
Figures



Comment in
-
ICAM-1: role in inflammation and in the regulation of vascular permeability.Am J Physiol Heart Circ Physiol. 2008 Sep;295(3):H926-H927. doi: 10.1152/ajpheart.00779.2008. Epub 2008 Aug 8. Am J Physiol Heart Circ Physiol. 2008. PMID: 18689494 Free PMC article. No abstract available.
References
-
- Clark PR, Manes TD, Pober JS, Kluger MS. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol 127: 762–774, 2007. - PubMed
-
- Czabanka M, Peter C, Martin E, Walther A. Microcirculatory endothelial dysfunction during endotoxemia—insights into pathophysiology, pathologic mechanisms and clinical relevance. Curr Vasc Pharmacol 5: 266–275, 2007. - PubMed
-
- Dunne JL, Ballantyne CM, Beaudet AL, Ley K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood 99: 336–341, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

