Specialized podosome- or invadopodia-like structures (PILS) for focal trabecular meshwork extracellular matrix turnover
- PMID: 18641286
- PMCID: PMC2683617
- DOI: 10.1167/iovs.07-1666
Specialized podosome- or invadopodia-like structures (PILS) for focal trabecular meshwork extracellular matrix turnover
Abstract
Purpose: There are distinctive areas of colocalization of matrix metalloproteinase (MMP)-2 and -14 on trabecular meshwork (TM) cells that resemble podosomes or invadopodia. Studies were conducted to determine whether TM cells exhibit podosome- or invadopodia-like structures (PILS) and whether they produce focal extracellular matrix (ECM) turnover.
Methods: Porcine and human TM cells and perfused anterior segment organ cultures were studied. Localization of PILS components on TM cells and in sections from anterior segments was determined by immunohistochemistry and confocal microscopy. Cells were grown on type I collagen labeled with fluorescein isothiocyanate (FITC) for degradation analysis. Confocal time lapse images were taken of labeled TM cells on FITC-collagen.
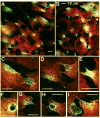
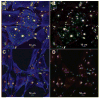
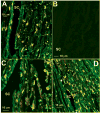
Results: Immunostaining for MMP-2, MMP-14, and the typical PILS components cortactin, caldesmon, alpha-actinin, N-WASP, Arp-3, and cdc42 colocalized on these distinctive structures. Integrin-alphaV and -beta1, fibronectin, and versican colocalized with PILS components. TM cells on FITC-conjugated collagen developed focal regions of degradation. Time-lapse imaging showed dramatic and controlled movement of TM cell processes during this ECM degradation and fragment internalization. MMP-2, MMP-14, and cortactin colocalized at regions that appear to be PILS on cells within the outflow pathway in sections of human anterior segments.
Conclusions: TM cells exhibit areas where PILS components colocalize with MMP-2 and -14. Similar structures are found in sections, suggesting that PILS occur in situ in the outflow pathway. The collagen degradation suggests that PILS may serve as focal sites for targeted ECM turnover, an event linked to modifications of aqueous outflow resistance and intraocular pressure homeostasis.
Conflict of interest statement
Disclosure:
Figures





References
-
- Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328:1097–1106. - PubMed
-
- Shields MB. Textbook of Glaucoma. 4th. Baltimore: Williams & Wilkins; 1998.
-
- Boland MV, Quigley HA. Risk factors and open-angle glaucoma: classification and application. J Glaucoma. 2007;16:406–418. - PubMed
-
- Bill A, Maepea O. Mechanisms and routes of aqueous humor drainage. In: Albert DM, Jakotiec FM, editors. Principles and Practices of Ophthalmology. Philadelphia: WB Saunders; 1994. pp. 206–226.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

