An endogenous prostaglandin enhances environmental phthalate-induced apoptosis in bone marrow B cells: activation of distinct but overlapping pathways
- PMID: 18641309
- PMCID: PMC2494875
- DOI: 10.4049/jimmunol.181.3.1728
An endogenous prostaglandin enhances environmental phthalate-induced apoptosis in bone marrow B cells: activation of distinct but overlapping pathways
Abstract
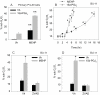
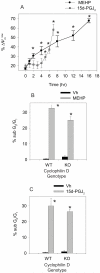
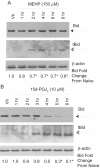
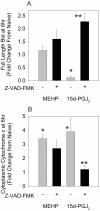
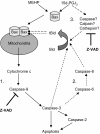
Phthalate esters are ubiquitous environmental contaminants that are produced for a variety of common industrial and commercial purposes. We have shown that mono-(2-ethylhexyl) phthalate (MEHP), the toxic metabolite of di-(2-ethylhexyl) phthalate, induces bone marrow B cell apoptosis that is enhanced in the presence of the endogenous prostaglandin 15-deoxy-Delta((12, 14))-PGJ(2) (15d-PGJ(2)). Here, studies were performed to determine whether 15d-PGJ(2)-mediated enhancement of MEHP-induced apoptosis represents activation of an overlapping or complementary apoptosis pathway. MEHP and 15d-PGJ(2) induced significant apoptosis within 8 and 5 h, respectively, in a pro/pre-B cell line and acted cooperatively to induce apoptosis in primary pro-B cells. Apoptosis induced with each chemical was accompanied by activation of a combination of initiator caspases (caspases-2, -8, and -9) and executed by caspase-3. Apoptosis induced with MEHP and 15d-PGJ(2) was reduced in APAF1 null primary pro-B cells and accompanied by alteration of mitochondrial membranes, albeit with different kinetics, indicating an intrinsically activated apoptosis pathway. Significant Bax translocation to the mitochondria supports its role in initiating release of cytochrome c. Both chemicals induced Bid cleavage, a result consistent with a truncated Bid-mediated release of cytochrome c in an apoptosis amplification feedback loop; however, significantly more Bid was cleaved following 15d-PGJ(2) treatment, potentially differentiating the two pathways. Indeed, Bid cleavage and cytochrome c release following 15d-PGJ(2) but not MEHP treatment was profoundly inhibited by Z-VAD-FMK, suggesting that 15d-PGJ(2) activates apoptosis via two pathways, Bax mobilization and protease-dependent Bid cleavage. Thus, endogenous 15d-PGJ(2)-mediated enhancement of environmental chemical-induced apoptosis represents activation of an overlapping but distinct signaling pathway.
Figures




References
-
- Koch HM, Drexler H, Angerer J. An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int. J. Hyg. Environ. Health. 2003;206:77. - PubMed
-
- Wittassek M, Heger W, Koch HM, Becker K, Angerer J, Kolossa-Gehring M. Daily intake of di(2-ethylhexyl)phthalate (DEHP) by German children -- A comparison of two estimation models based on urinary DEHP metabolite levels. Int J Hyg Environ Health. 2007;210:35. - PubMed
-
- Plonait SL, Nau H, Maier RF, Wittfoht W, Obladen M. Exposure of newborn infants to di-(2-ethylhexyl)-phthalate and 2-ethylhexanoic acid following exchange transfusion with polyvinylchloride catheters. Transfusion. 1993;33:598. - PubMed
-
- Tickner JA, Schettler T, Guidotti T, McCally M, Rossi M. Health risks posed by use of di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: a critical review. Am. J. Ind. Med. 2001;39:100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

