Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation
- PMID: 18641321
- PMCID: PMC2587319
- DOI: 10.4049/jimmunol.181.3.1835
Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation
Abstract
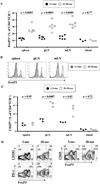
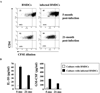
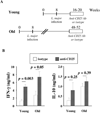
Declines in immune function are well described in the elderly and are considered to contribute significantly to the disease burden in this population. Regulatory T cells (T(regs)), a CD4(+) T cell subset usually characterized by high CD25 expression, control the intensity of immune responses both in rodents and humans. However, because CD25 expression does not define all T(regs), especially in aged hosts, we characterized T(regs) by the expression of FOXP3, a transcription factor crucial for T(reg) differentiation and function. The proportion of FOXP3(+)CD4(+) T(regs) increased in the blood of the elderly and the lymphoid tissues of aged mice. The expression of functional markers, such as CTLA-4 and GITR, was either preserved or increased on FOXP3(+) T(regs) from aged hosts, depending on the tissue analyzed. In vitro depletion of peripheral T(regs) from elderly humans improves effector T cell responses in most subjects. Importantly, T(regs) from old FoxP3-GFP knock-in mice were suppressive, exhibiting a higher level of suppression per cell than young T(regs). The increased proportion of T(regs) in aged mice was associated with the spontaneous reactivation of chronic Leishmania major infection in old mice, likely because old T(regs) efficiently suppressed the production of IFN-gamma by effector T cells. Finally, in vivo depletion of T(regs) in old mice attenuated disease severity. Accumulation of functional T(regs) in aged hosts could therefore play an important role in the frequent reactivation of chronic infections that occurs in aging. Manipulation of T(reg) numbers and/or activity may be envisioned to enhance the control of infectious diseases in this fragile population.
Figures






Similar articles
-
Suppression of glomerulonephritis in NZB/NZW lupus prone mice by adoptive transfer of ex vivo expanded regulatory T cells.PLoS One. 2009 Jun 24;4(6):e6031. doi: 10.1371/journal.pone.0006031. PLoS One. 2009. PMID: 19551149 Free PMC article.
-
A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection.J Immunol. 2005 May 1;174(9):5444-55. doi: 10.4049/jimmunol.174.9.5444. J Immunol. 2005. PMID: 15845457
-
Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity.J Exp Med. 2004 Jul 19;200(2):201-10. doi: 10.1084/jem.20040298. J Exp Med. 2004. PMID: 15263027 Free PMC article.
-
Alterations in regulatory T-cells: rediscovered pathways in immunotoxicology.J Immunotoxicol. 2011 Oct-Dec;8(4):251-7. doi: 10.3109/1547691X.2011.598885. Epub 2011 Aug 17. J Immunotoxicol. 2011. PMID: 21848365 Free PMC article. Review.
-
Regulatory T cells in arterivirus and coronavirus infections: do they protect against disease or enhance it?Viruses. 2012 May;4(5):833-46. doi: 10.3390/v4050833. Epub 2012 May 15. Viruses. 2012. PMID: 22754651 Free PMC article. Review.
Cited by
-
Autoreactive Peripheral Blood T Helper Cell Responses in Bullous Pemphigoid and Elderly Patients With Pruritic Disorders.Front Immunol. 2021 Mar 25;12:569287. doi: 10.3389/fimmu.2021.569287. eCollection 2021. Front Immunol. 2021. PMID: 33841390 Free PMC article.
-
Age matters: Young T lymphocytes offer better protection from myeloma proliferation.Immun Ageing. 2013 Feb 18;10(1):5. doi: 10.1186/1742-4933-10-5. Immun Ageing. 2013. PMID: 23419047 Free PMC article.
-
Regulatory KIR+ RA+ T cells accumulate with age and are highly activated during viral respiratory disease.Aging Cell. 2021 Jun;20(6):e13372. doi: 10.1111/acel.13372. Epub 2021 May 27. Aging Cell. 2021. PMID: 34043881 Free PMC article.
-
Immunity, cancer and aging: lessons from mouse models.Aging Dis. 2011 Dec;2(6):512-23. Epub 2011 Dec 2. Aging Dis. 2011. PMID: 22396898 Free PMC article.
-
Thymic Aging May Be Associated with COVID-19 Pathophysiology in the Elderly.Cells. 2021 Mar 12;10(3):628. doi: 10.3390/cells10030628. Cells. 2021. PMID: 33808998 Free PMC article. Review.
References
-
- Effros RB. Long-term immunological memory against viruses. Mech Ageing Dev. 2000;121:161–171. - PubMed
-
- Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, Abrutyn E. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37:427–439. - PubMed
-
- Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2:659–666. - PubMed
-
- Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25:599–604. - PubMed
-
- Rytel MW. Effect of age on viral infections: possible role of interferon. J Am Geriatr Soc. 1987;35:1092–1099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

