CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals
- PMID: 18641350
- PMCID: PMC4161464
- DOI: 10.4049/jimmunol.181.3.2111
CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals
Abstract
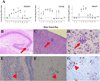
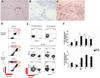
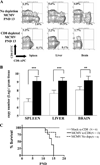
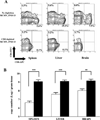
Human CMV infection of the neonatal CNS results in long-term neurologic sequelae. To define the pathogenesis of fetal human CMV CNS infections, we investigated mechanisms of virus clearance from the CNS of neonatal BALB/c mice infected with murine CMV (MCMV). Virus titers peaked in the CNS between postnatal days 10-14 and infectious virus was undetectable by postnatal day 21. Congruent with virus clearance was the recruitment of CD8(+) T cells into the CNS. Depletion of CD8(+) T cells resulted in death by postnatal day 15 in MCMV-infected animals and increased viral loads in the liver, spleen, and the CNS, suggesting an important role for these cells in the control of MCMV replication in the newborn brain. Examination of brain mononuclear cells revealed that CD8(+) T cell infiltrates expressed high levels of CD69, CD44, and CD49d. IE1(168)-specific CD8(+) T cells accumulated in the CNS and produced IFN-gamma and TNF-alpha but not IL-2 following peptide stimulation. Moreover, adoptive transfer of brain mononuclear cells resulted in decreased virus burden in immunodepleted MCMV-infected syngeneic mice. Depletion of the CD8(+) cell population following transfer eliminated control of virus replication. In summary, these results show that functionally mature virus-specific CD8(+) T cells are recruited to the CNS in mice infected with MCMV as neonates.
Figures




Similar articles
-
T cell-mediated restriction of intracerebral murine cytomegalovirus infection displays dependence upon perforin but not interferon-gamma.J Neurovirol. 2005 Jul;11(3):274-80. doi: 10.1080/13550280590952808. J Neurovirol. 2005. PMID: 16036807 Free PMC article.
-
Glucocorticoid treatment of MCMV infected newborn mice attenuates CNS inflammation and limits deficits in cerebellar development.PLoS Pathog. 2013 Mar;9(3):e1003200. doi: 10.1371/journal.ppat.1003200. Epub 2013 Mar 7. PLoS Pathog. 2013. PMID: 23505367 Free PMC article.
-
Hyperexpansion of Functional Viral-Specific CD8+ T Cells in Lymphopenia-Associated MCMV Pneumonitis.Viral Immunol. 2015 Jun;28(5):255-64. doi: 10.1089/vim.2015.0005. Epub 2015 May 18. Viral Immunol. 2015. PMID: 26046830 Free PMC article.
-
CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation.J Virol. 2006 Nov;80(21):10436-56. doi: 10.1128/JVI.01248-06. Epub 2006 Aug 23. J Virol. 2006. PMID: 16928768 Free PMC article.
-
Memory T cells persisting in the brain following MCMV infection induce long-term microglial activation via interferon-γ.J Neurovirol. 2011 Oct;17(5):424-37. doi: 10.1007/s13365-011-0042-5. Epub 2011 Jul 29. J Neurovirol. 2011. PMID: 21800103 Free PMC article.
Cited by
-
Immune surveillance of cytomegalovirus in tissues.Cell Mol Immunol. 2024 Sep;21(9):959-981. doi: 10.1038/s41423-024-01186-2. Epub 2024 Aug 12. Cell Mol Immunol. 2024. PMID: 39134803 Free PMC article. Review.
-
The Current Challenges in Developing Biological and Clinical Predictors of Congenital Cytomegalovirus Infection.Int J Mol Sci. 2021 Dec 15;22(24):13487. doi: 10.3390/ijms222413487. Int J Mol Sci. 2021. PMID: 34948284 Free PMC article. Review.
-
The Neonatal CD8+ T Cell Repertoire Rapidly Diversifies during Persistent Viral Infection.J Immunol. 2016 Feb 15;196(4):1604-16. doi: 10.4049/jimmunol.1501867. Epub 2016 Jan 13. J Immunol. 2016. PMID: 26764033 Free PMC article.
-
Murine Cytomegalovirus Infection Induces Susceptibility to EAE in Resistant BALB/c Mice.Front Immunol. 2017 Feb 27;8:192. doi: 10.3389/fimmu.2017.00192. eCollection 2017. Front Immunol. 2017. PMID: 28289417 Free PMC article.
-
Histone demethylase UTX/KDM6A enhances tumor immune cell recruitment, promotes differentiation and suppresses medulloblastoma.Cancer Lett. 2021 Feb 28;499:188-200. doi: 10.1016/j.canlet.2020.11.031. Epub 2020 Nov 27. Cancer Lett. 2021. PMID: 33253789 Free PMC article.
References
-
- McCracken GH, Jr, Shinefield HM, Cobb K, Rausen AR, Dische R, Eichenwald HF. Congenital cytomegalic inclusion disease. A longitudinal study of 20 patients. Am J Dis Child. 1969;117:522–539. - PubMed
-
- Weller TH, Hanshaw JB. Virologic and clinical observations on cytomegalic inclusion disease. N Engl J Med. 1962;266:1233–1244. - PubMed
-
- Williamson WD, Desmond MM, LaFevers N, Taber LH, Catlin FI, Weaver TG. Symptomatic congenital cytomegalovirus. Disorders of language, learning, and hearing. Am J Dis Child. 1982;136:902–905. - PubMed
-
- Stagno S. Cytomegalovirus. In: Remington J, Klein JO, editors. Infectious Diseases of Fetus and Newborn. Philadelphia: W.B. Saunders Company; 2001.
-
- Weller TH. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med. 1971;285:203–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

