Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance
- PMID: 18641351
- PMCID: PMC3319690
- DOI: 10.4049/jimmunol.181.3.2124
Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance
Abstract
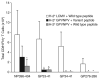
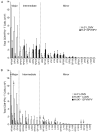
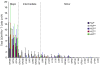
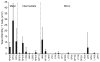
The primary CD8(+) T cell response of C57BL/6J mice against the 28 known epitopes of lymphocytic choriomeningitis virus (LCMV) is associated with a clear immunodominance hierarchy whose mechanism has yet to be defined. To evaluate the role of epitope competition in immunodominance, we manipulated the number of CD8(+) T cell epitopes that could be recognized during LCMV infection. Decreasing epitope numbers, using a viral variant lacking dominant epitopes or C57BL/6J mice lacking H-2K(b), resulted in minor response increases for the remaining epitopes and no new epitopes being recognized. Increasing epitope numbers by using F(1) hybrid mice, delivery by recombinant vaccinia virus, or epitope delivery as a pool in IFA maintained the overall response pattern; however, changes in the hierarchy did become apparent. MHC binding affinity of these epitopes was measured and was found to not strictly predict the hierarchy since in several cases similarly high binding affinities were associated with differences in immunodominance. In these instances the naive CD8(+) T cell precursor frequency, directly measured by tetramer staining, correlated with the response hierarchy seen after LCMV infection. Finally, we investigated an escape mutant of the dominant GP33-41 epitope that elicited a weak response following LCMV variant virus infection. Strikingly, dominance loss likely reflects a substantial reduction in frequencies of naive precursors specific for this epitope. Thus, our results indicate that an intrinsic property of the epitope (MHC binding affinity) and an intrinsic property of the host (naive precursor frequency) jointly dictate the immunodominance hierarchy of CD8(+) T cell responses.
Conflict of interest statement
Figures



References
-
- Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. - PubMed
-
- Yewdell JW. Confronting complexity: real-world immunodominance in antiviral CD8+T cell responses. Immunity. 2006;25:533–543. - PubMed
-
- Pang KC, Sanders MT, Monaco JJ, Doherty PC, Turner SJ, Chen W. Immunoproteasome subunit deficiencies impact differentially on two immunodominant influenza virus-specific CD8+ T cell responses. J Immunol. 2006;177:7680–7688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

