Bcl-xL and UVRAG cause a monomer-dimer switch in Beclin1
- PMID: 18641390
- PMCID: PMC3258859
- DOI: 10.1074/jbc.M804723200
Bcl-xL and UVRAG cause a monomer-dimer switch in Beclin1
Abstract
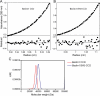
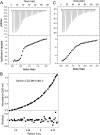
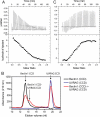
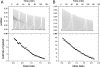
Beclin1 has a key regulatory role in the initiation of autophagy and is a tumor suppressor. We have examined the interplay between viral or human Bcl-2-like proteins and UVRAG and their opposite effects on Beclin1. We show that Beclin1 forms a dimer in solution via its coiled-coil domain both in vivo and in vitro. Viral Bcl-2 binds independently to two sites on the Beclin1 dimer, one with high affinity and one with lower affinity, whereas human Bcl-x(L) binds both sites equally with relatively low affinity. UVRAG disrupts the Beclin1-dimer interface, forming a heterodimer with Beclin1, suggesting that this is how UVRAG causes its effects on Beclin1 to activate autophagy. Both Bcl-2-like proteins reduce the affinity of UVRAG for Beclin1 approximately 4-fold, suggesting that they stabilize the Beclin1 dimer. Moreover, coimmunoprecipitation assays show that UVRAG substantially reduces Beclin1 dimerization in vivo. These data explain the concentration-dependent interplay between Bcl-2, UVRAG, and Beclin1, as both tumor suppressors, UVRAG and Beclin1, have single-copy mutations in human cancers. Furthermore, our data suggest that an alternative strategy for developing anti-cancer compounds would be to disrupt the Beclin1-dimer interface.
Figures

Similar articles
-
Imperfect interface of Beclin1 coiled-coil domain regulates homodimer and heterodimer formation with Atg14L and UVRAG.Nat Commun. 2012 Feb 7;3:662. doi: 10.1038/ncomms1648. Nat Commun. 2012. PMID: 22314358 Free PMC article.
-
Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG.Nat Cell Biol. 2006 Jul;8(7):688-99. doi: 10.1038/ncb1426. Epub 2006 Jun 25. Nat Cell Biol. 2006. PMID: 16799551
-
Beclin 1 self-association is independent of autophagy induction by amino acid deprivation and rapamycin treatment.J Cell Biochem. 2010 Aug 1;110(5):1262-71. doi: 10.1002/jcb.22642. J Cell Biochem. 2010. PMID: 20564222
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
UVRAG: a new player in autophagy and tumor cell growth.Autophagy. 2007 Jan-Feb;3(1):69-71. doi: 10.4161/auto.3437. Epub 2007 Jan 27. Autophagy. 2007. PMID: 17106237 Review.
Cited by
-
Conformational flexibility of BECN1: Essential to its key role in autophagy and beyond.Protein Sci. 2016 Oct;25(10):1767-85. doi: 10.1002/pro.2984. Epub 2016 Aug 13. Protein Sci. 2016. PMID: 27414988 Free PMC article. Review.
-
New Insights Into Beclin-1: Evolution and Pan-Malignancy Inhibitor Activity.Adv Cancer Res. 2018;137:77-114. doi: 10.1016/bs.acr.2017.11.002. Epub 2017 Dec 27. Adv Cancer Res. 2018. PMID: 29405978 Free PMC article. Review.
-
The BECN1 N-terminal domain is intrinsically disordered.Autophagy. 2016;12(3):460-71. doi: 10.1080/15548627.2016.1140292. Autophagy. 2016. PMID: 27046249 Free PMC article.
-
The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond.Trends Cell Biol. 2010 Jun;20(6):355-62. doi: 10.1016/j.tcb.2010.03.002. Epub 2010 Mar 29. Trends Cell Biol. 2010. PMID: 20356743 Free PMC article. Review.
-
Bcl-xL as a Modulator of Senescence and Aging.Int J Mol Sci. 2021 Feb 3;22(4):1527. doi: 10.3390/ijms22041527. Int J Mol Sci. 2021. PMID: 33546395 Free PMC article. Review.
References
-
- Levine, B., and Klionsky, D. J. (2004) Dev. Cell 6 463-477 - PubMed
-
- Shimizu, S., Kanaseki, T., Mizushima, N., Mizuta, T., Arakawa-Kobayashi, S., Thompson, C. B., and Tsujimoto, Y. (2004) Nat. Cell Biol. 6 1221-1228 - PubMed
-
- Yu, L., Alva, A., Su, H., Dutt, P., Freundt, E., Welsh, S., Baehrecke, E. H., and Lenardo, M. J. (2004) Science 304 1500-1502 - PubMed
-
- Aita, V. M., Liang, X. H., Murty, V. V., Pincus, D. L., Yu, W., Cayanis, E., Kalachikov, S., Gilliam, T. C., and Levine, B. (1999) Genomics 59 59-65 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

