Corollary discharge across the animal kingdom
- PMID: 18641666
- PMCID: PMC5153363
- DOI: 10.1038/nrn2457
Corollary discharge across the animal kingdom
Abstract
Our movements can hinder our ability to sense the world. Movements can induce sensory input (for example, when you hit something) that is indistinguishable from the input that is caused by external agents (for example, when something hits you). It is critical for nervous systems to be able to differentiate between these two scenarios. A ubiquitous strategy is to route copies of movement commands to sensory structures. These signals, which are referred to as corollary discharge (CD), influence sensory processing in myriad ways. Here we review the CD circuits that have been uncovered by neurophysiological studies and suggest a functional taxonomic classification of CD across the animal kingdom. This broad understanding of CD circuits lays the groundwork for more challenging studies that combine neurophysiology and psychophysics to probe the role of CD in perception.
Figures


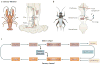
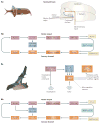
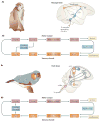
References
-
- Poincaré H. Science et methode. Flammarion; Paris: 1897.
-
- Holst EV, Mittelstaedt H. The reafference principle. Naturwissenschaften. 1950;37:464–467.
-
- Sperry R. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–489. References 2 and 3 are two groundbreaking papers that were published independently and nearly simultaneously. They were the first to propose in a rigorous manner, and with supporting experimental evidence, that motor-to-sensory feedback has a critical role in regulating animal behaviour. - PubMed
-
- Cullen KE. Sensory signals during active versus passive movement. Curr Opin Neurobiol. 2004;14:698–706. - PubMed
-
- Poulet JF, Hedwig B. New insights into corollary discharges mediated by identified neural pathways. Trends Neurosci. 2007;30:14–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

