Regulating the angiogenic balance in tissues
- PMID: 18642446
- PMCID: PMC3121332
- DOI: 10.4161/cc.7.13.6240
Regulating the angiogenic balance in tissues
Abstract
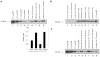
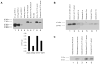
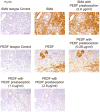
A balance between angiogenesis inducers and inhibitors in the microenvironment controls the rate of new blood vessel formation. We hypothesized that fibroblasts, an important cellular constituent of the tissue stroma, secrete molecules that contribute to this balance. We further hypothesized that fibroblasts secrete molecules that promote angiogenesis when they are in a proliferative state and molecules that inhibit angiogenesis when they are not actively cycling (quiescent). Microarray analysis revealed that angiogenesis inducers and inhibitors are regulated as fibroblasts transition into a quiescent state and reenter the cell cycle in response to changes in serum. To assess whether changes in transcript levels result in changes in the levels of secreted proteins, we collected conditioned medium from proliferating and quiescent fibroblasts and performed immunoblotting for selected proteins. Secreted protein levels of the angiogenesis inhibitor pigment epithelium derived factor (PEDF) were higher in quiescent than proliferating fibroblasts. Conversely, proliferating fibroblasts secreted increased levels of the angiogenesis inducer vascular endothelial growth factor-C (VEGF-C). For the angiogenesis inhibitor thrombospondin-2, quiescent cells secreted a prominent 160 kDa form in addition to the 200 kDa form secreted by proliferating and restimulated fibroblasts. Using immunohistochemistry we discovered that fibroblasts surround blood vessels and that the angiogenesis inhibitor PEDF is expressed by quiescent fibroblasts in uterine tissue, supporting a role for PEDF in maintaining quiescence of the vasculature. This work takes a new approach to the study of angiogenesis by examining the expression of multiple angiogenesis regulators secreted from a key stromal cell, the fibroblast.
Figures






References
-
- Mathews LS, Hammer RE, Behringer RR, D'Ercole AJ, Bell GI, Brinster RL, Palmiter RD. Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology. 1988;123:2827–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
