Total synthesis and biological mode of action of largazole: a potent class I histone deacetylase inhibitor
- PMID: 18642817
- PMCID: PMC3090445
- DOI: 10.1021/ja8033763
Total synthesis and biological mode of action of largazole: a potent class I histone deacetylase inhibitor
Abstract
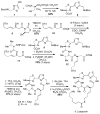
The efficient total synthesis of the recently described natural substance largazole (1) and its active metabolite largazole thiol (2) is described. The synthesis required eight linear steps and proceeded in 37% overall yield. It is demonstrated that largazole is a pro-drug that is activated by removal of the octanoyl residue from the 3-hydroxy-7-mercaptohept-4-enoic acid moiety to generate the active metabolite 2, which is an extraordinarily potent Class I histone deacetylase inhibitor. Synthetic largazole and 2 have been evaluated side-by-side with FK228 and SAHA for inhibition of HDACs 1, 2, 3, and 6. Largazole and largazole thiol were further assayed for cytotoxic activity against a panel of chemoresistant melanoma cell lines, and it was found that largazole is substantially more cytotoxic than largazole thiol; this difference is attributed to differences in the cell permeability of the two substances.
Figures





References
-
-
Taori K, Paul VJ, Luesch H. J Am Chem Soc. 2008;130:1806–1807. subsequent to the submission of the present manuscript on May 6, 2008, Luesch, et al. independently published a distinct synthesis of largazole; see: Ying Y, Taori K, Kim H, Homg J, Luesch H. J Am Chem Soc. 2008;130:0000. (published on-line May 29, 2008)
-
-
- Fujisawa Pharmaceutical Co., Ltd. 03141296. Jpn Kokai Tokkyo Koho JP. 1991
- Ueda H, Nakajima H, Hori Y, Fujita T, Nishimura M, Goto T, Okuhara M. J Antibiot. 1994;47:301. - PubMed
- Shigematsu N, Ueda H, Takase S, Tanaka H. J Antibiot. 1994;47:311. - PubMed
- Ueda H, Manda T, Matsumoto S, Mukumoto S, Nishigaki F, Kawamura I, Shimomura K. J Antibiot. 1994;47:315. - PubMed
-
- Masuoka Y, Nagai A, Shin-ya K, Furihata K, Nagai K, Suzuki K, Hayakawa Y, Seto H. Tetrahedron Lett. 2001;42:41. - PubMed
-
- Townsend PA, Crabb SJ, Davidson SM, Johnson PWM, Packham G, Ganesan A. The bicyclic depsipeptide family of histone deacetylase inhibitors. In: Schreiber SL, Kapoor TM, Wess G, editors. Chemical Biology. Wiley-VCH Verlag GmbH & Co; Weinheim, Germany: 2007. pp. 693–720.
-
-
Ref. 1b substantially corrected the error in ref. 1a with respect to the novelty of the (S)-3-hydroxy-7-mercaptohept-4-enoic acid moiety and also reported IC50 HDAC inhibitory data for Largazole.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

