A versatile cyclodehydration reaction for the synthesis of isoquinoline and beta-carboline derivatives
- PMID: 18642832
- PMCID: PMC2692836
- DOI: 10.1021/ol801264u
A versatile cyclodehydration reaction for the synthesis of isoquinoline and beta-carboline derivatives
Abstract
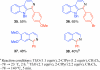
The direct conversion of various amides to isoquinoline and beta-carboline derivatives via mild electrophilic amide activation, with trifluoromethanesulfonic anhydride in the presence of 2-chloropyridine, is described. Low-temperature amide activation followed by cyclodehydration upon warming provides the desired products with short overall reaction times. The successful use of nonactivated and halogenated phenethylene derived amides, N-vinyl amides, and optically active substrates is noteworthy.
Figures
References
-
- Bischler A, Napieralski B. Ber. 1893;26:1903.
- Whaley WM, Govindachari TR. Org. React. 1951;6:74.
-
-
For representative reports, see: Tóth J, Nedves A, Dancsó A, Blaskó G, Töke L, Nyerges M. Synthesis. 2007:1003.Spaggiari A, Davoli P, Blaszczak LC, Prati F. Synlett. 2005:661.Banwell MG, Bissett BD, Busato S, Cowden CJ, Hockless DCR, Holman JW, Read RW, Wu AW. J. Chem. Soc., Chem. Commun. 1995:2551.Larsen RD, Reamer RA, Corley EG, Davis P, Grabowski EJJ, Reider PJ, Shinkai I. J. Org. Chem. 1991;56:6034.Hendrickson JB, Schwartzman SM. Tetrahedron Lett. 1975;16:277.
-
-
-
For reviews on isoquinolines and their reduced derivatives, see Jones G. In: Comprehensive Heterocyclic Chemistry II. Katritzky AR, Rees CW, Scriven EFV, McKillop A, editors. Vol. 5. Pergamon; Oxford: 1996. p. 167.Bentley KW. Nat. Prod. Rep. 2006;23:444.Kartsev VG. Med. Chem. Res. 2004;13:325.Chrzanowska M, Rozwadowska MD. Chem. Rev. 2004;104:3341.Joule JA, Mills K. Heterocyclic Chemistry. 4th ed. Blackwell Science Ltd.; Cambridge MA: 2000. p. 121.Rozwadowska MD. Heterocycles. 1994;39:903. For a review on β-carbolines and their reduced derivatives, see Love BE. Org. Prep. Proced. Int. 1996;28:3.
-
-
-
For recent advances in synthesis of N-vinyl and N-aryl amides, see: Muci AR, Buchwald SL. Top. Curr. Chem. 2002;219:131.Hartwig JF. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, editor. Wiley-Interscience; New York: 2002. p. 1051.Beletskaya IP, Cheprakov AV. Coordin. Chem. Rev. 2004;248:2337.Dehli JR, Legros J, Bolm C. Chem. Commun. 2005:973.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases