Characterization of the peptidoglycan of vancomycin-susceptible Enterococcus faecium
- PMID: 18642854
- PMCID: PMC2742421
- DOI: 10.1021/bi8008032
Characterization of the peptidoglycan of vancomycin-susceptible Enterococcus faecium
Abstract
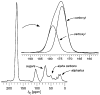
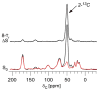
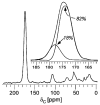
Vancomycin and other antibacterial glycopeptide analogues target the cell wall and affect the enzymatic processes involved with cell-wall biosynthesis. Understanding the structure and organization of the peptidoglycan is the first step in establishing the mode of action of these glycopeptides. We have used solid-state NMR to determine the relative concentrations of stem-links (64%), bridge-links (61%), and cross-links (49%) in the cell walls of vancomycin-susceptible Enterococcus faecium (ATTC 49624). Furthermore, we have determined that in vivo only 7% of the peptidoglycan stems terminate in d-Ala- d-Ala, the well-known vancomycin-binding site. Presumably, d-Ala- d-Ala is cleaved from uncross-linked stems in mature peptidoglycan by an active carboxypeptidase. We believe that most of the few pentapeptide stems ending in d-Ala- d-Ala occur in the template and nascent peptidoglycan strands that are crucial for cell-wall biosynthesis.
Figures







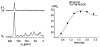


References
-
- Mainardi JL, Villet R, Bugg TD, Mayer C, Arthur M. Evolution of peptidoglycan biosynthesis under the selective pressure of antibiotics in Gram-positive bacteria. FEMS Microbiol Rev. 2008;32:386–408. - PubMed
-
- Uttley AH, Collins CH, Naidoo J, George RC. Vancomycin-resistant enterococci. Lancet. 1988;1:57–58. - PubMed
-
- Kak VaC, Joseph W. Acquired Antibiotic Resistances in Enterococci. ASM Press; Washington, DC: 2002.
-
- Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis. 2006;42:S25–S34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

