Heteroligated supramolecular coordination complexes formed via the halide-induced ligand rearrangement reaction
- PMID: 18642933
- PMCID: PMC8191499
- DOI: 10.1021/ar800025w
Heteroligated supramolecular coordination complexes formed via the halide-induced ligand rearrangement reaction
Abstract
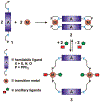
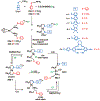
Supramolecular coordination chemistry allows researchers to synthesize higher-order structures that approach the nanoscale dimensions of small enzymes. Frequently, such structures have highly symmetric macrocyclic square or cage shapes. To build functional structures that mimic the complex recognition, catalytic, and allosteric properties of enzymes, researchers must do more than synthesize highly symmetric nanoscale structures. They must also simultaneously incorporate different functionalities into these structures and learn how to regulate their relative arrangement with respect to each other. Designing such heteroligated coordination complexes remains a significant challenge for supramolecular chemists. This Account focuses on the discovery and development of a novel supramolecular reaction known as the halide-induced ligand rearrangement (HILR) reaction. Two hemilabile ligands with different binding strengths combine with d(8) transition metal precursors that contain halide ions. The reaction spontaneously results in heteroligated complexes and is highly modular and general. Indeed, it not only can be used to prepare tweezer complexes but also allows for the rapid and quantitative formation of heteroligated macrocyclic triple-decker/step and rectangular box complexes from a variety of different ligands and transition metal ions. The relative arrangement between functional groups A and B in these structures can be regulated in situ using small ancillary ligands such as halides, CO, and nitriles. Based on this reaction, zinc- and magnesium-porphyrin moieties can be incorporated into heteroligated macrocyclic or tweezer scaffolds. These examples demonstrate the convergent and cofacial assembly of functional sites that are known to be involved in numerous processes in enzymes. They also show how the relative spatial and lateral distances of these sites can be varied, in many cases reversibly. Researchers can use such complexes to study a wide range of enzymatic processes, including catalysis, molecular recognition, electron transfer, and allosteric signal transfer.
Figures
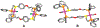

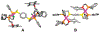
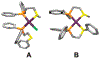
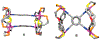
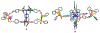

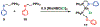







References
-
- Farrell JR; Mirkin CA; Guzei IA; Liable-Sands LM; Rheingold AL The Weak-Link Approach to the Synthesis of Inorganic Macrocyles. Angew. Chem., Int. Ed 1998, 37, 465–467. - PubMed
-
- Caulder DL; Raymond RN The Rational Design of High Symmetry Coordination Clusters. J. Chem. Soc., Dalton Trans 1999, 1185–1200.
-
- Caulder DL; Raymond RN Supermolecules by Design. Acc. Chem. Res 1999, 32, 975–982.
-
- Holliday BJ; Mirkin CA Strategies for the Construction of Supramolecular Compounds through Coordination Chemistry. Angew. Chem., Int. Ed 2001, 40, 2022–2043. - PubMed
-
- Seidel SR; Stang PJ High-Symmetry Coordination Cages via Self-Assembly. Acc. Chem. Res 2002, 35, 972–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

