The clinical pharmacology of intranasal l-methamphetamine
- PMID: 18644153
- PMCID: PMC2496900
- DOI: 10.1186/1472-6904-8-4
The clinical pharmacology of intranasal l-methamphetamine
Abstract
Background: We studied the pharmacology of l-methamphetamine, the less abused isomer, when used as a nasal decongestant.
Methods: 12 subjects self-administered l-methamphetamine from a nonprescription inhaler at the recommended dose (16 inhalations over 6 hours) then at 2 and 4 (32 and 64 inhalations) times this dose. In a separate session intravenous phenylephrine (200 microg) and l-methamphetamine (5 mg) were given to define alpha agonist pharmacology and bioavailability. Physiological, cardiovascular, pharmacokinetic, and subjective effects were measured.
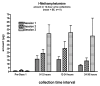
Results: Plasma l-methamphetamine levels were often below the level of quantification so bioavailability was estimated by comparing urinary excretion of the intravenous and inhaled doses, yielding delivered dose estimates of 74.0 +/- 56.1, 124.7 +/- 106.6, and 268.1 +/- 220.5 microg for ascending exposures (mean 4.2 +/- 3.3 microg/inhalation). Physiological changes were minimal and not dose-dependent. Small decreases in stroke volume and cardiac output suggesting mild cardiodepression were seen.
Conclusion: Inhaled l-methamphetamine delivered from a non-prescription product produced minimal effects but may be a cardiodepressant.
Figures
References
-
- Gal J. Amphetamines in nasal inhalers. J Toxicol Clin Toxicol. 1982;19:517–8.
-
- Logan BK. Methamphetamine – effects on human performance and behavior. Forensic Sci Rev. 2002;14:134–151. - PubMed
-
- Cold, cough, allergy, bronchodilator, and antiasthmatic drug products for over-the-counter human use; amendment of final monograph for over-the-counter nasal decongestant drug products. Final rule. Fed Regist. 2005;70:58974–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical