Pre-clinical characterization of GMP grade CCL21-gene modified dendritic cells for application in a phase I trial in non-small cell lung cancer
- PMID: 18644162
- PMCID: PMC2507704
- DOI: 10.1186/1479-5876-6-38
Pre-clinical characterization of GMP grade CCL21-gene modified dendritic cells for application in a phase I trial in non-small cell lung cancer
Abstract
Background: Our previous studies have demonstrated that transduction of human dendritic cells (DC) with adenovirus encoding secondary lymphoid chemokine, CCL21, led to secretion of biologically active CCL21 without altering DC phenotype or viability. In addition, intratumoral injections of CCL21-transduced DC into established murine lung tumors resulted in complete regression and protective anti-tumor immunity. These results have provided the rationale to generate a clinical grade adenoviral vector encoding CCL-21 for ex vivo transduction of human DC in order to assess intratumoral administration in late stage human lung cancer.
Methods: In the current study, human monocyte-derived DC were differentiated by exposure to GM-CSF and IL-4 from cryopreserved mononuclear cells obtained from healthy volunteers. Transduction with clinical grade adenoviral vector encoding CCL21 (1167 viral particles per cell) resulted in secretion of CCL21 protein.
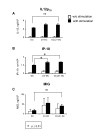
Results: CCL21 protein production from transduced DC was detected in supernatants (24-72 hours, 3.5-6.7 ng/4-5 x 10(6) cells). DC transduced with the clinical grade adenoviral vector were > 88% viable (n = 16), conserved their phenotype and maintained integral biological activities including dextran uptake, production of immunostimulatory cytokines/chemokines and antigen presentation. Furthermore, supernatant from CCL21-DC induced the chemotaxis of T2 cells in vitro.
Conclusion: Viable and biologically active clinical grade CCL21 gene-modified DC can be generated from cryopreserved PBMC.
Figures

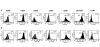

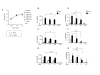

Similar articles
-
Overexpression of CCL-21/secondary lymphoid tissue chemokine in human dendritic cells augments chemotactic activities for lymphocytes and antigen presenting cells.Mol Cancer. 2003 Nov 2;2:35. doi: 10.1186/1476-4598-2-35. Mol Cancer. 2003. PMID: 14613584 Free PMC article.
-
Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity.Clin Cancer Res. 2004 Apr 15;10(8):2891-901. doi: 10.1158/1078-0432.ccr-03-0380. Clin Cancer Res. 2004. PMID: 15102698
-
Phase I Trial of Intratumoral Injection of CCL21 Gene-Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8+ T-cell Infiltration.Clin Cancer Res. 2017 Aug 15;23(16):4556-4568. doi: 10.1158/1078-0432.CCR-16-2821. Epub 2017 May 3. Clin Cancer Res. 2017. PMID: 28468947 Free PMC article. Clinical Trial.
-
In vivo manipulation of dendritic cell migration and activation to elicit antitumour immunity.Novartis Found Symp. 2004;256:241-54; discussion 254-69. doi: 10.1002/0470856734.ch18. Novartis Found Symp. 2004. PMID: 15027495 Review.
-
Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application.J Immunol Methods. 1999 Feb 1;223(1):1-15. doi: 10.1016/s0022-1759(98)00208-7. J Immunol Methods. 1999. PMID: 10037230 Review.
Cited by
-
New approaches to the development of adenoviral dendritic cell vaccines in melanoma.Curr Opin Investig Drugs. 2010 Dec;11(12):1399-408. Curr Opin Investig Drugs. 2010. PMID: 21154122 Free PMC article. Review.
-
Engineering Challenges and Opportunities in Autologous Cellular Cancer Immunotherapy.J Immunol. 2024 Jan 15;212(2):188-198. doi: 10.4049/jimmunol.2300642. J Immunol. 2024. PMID: 38166251 Free PMC article. Review.
-
Gene therapy for lung neoplasms.Clin Chest Med. 2011 Dec;32(4):865-85. doi: 10.1016/j.ccm.2011.08.006. Epub 2011 Oct 7. Clin Chest Med. 2011. PMID: 22054892 Free PMC article. Review.
-
The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis.Mol Cancer. 2015 Feb 10;14:35. doi: 10.1186/s12943-015-0306-4. Mol Cancer. 2015. PMID: 25744065 Free PMC article.
-
Chemokines, costimulatory molecules and fusion proteins for the immunotherapy of solid tumors.Immunotherapy. 2011 Nov;3(11):1317-40. doi: 10.2217/imt.11.115. Immunotherapy. 2011. PMID: 22053884 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Auberger J, Loeffler-Ragg J, Wurzer W, Hilbe W. Targeted therapies in Non-Small cell lung cancer: proven concepts and unfulfilled promises. Curr Canc Drug Targets. 2006;6:271–294. - PubMed
-
- Batra RK, Lin Y, Sharma S, Dohadwala M, Luo J, Pold M, Dubinett SM. Non-small cell lung cancer-derived soluble mediators enhance apoptosis in activated T lymphocytes through and NF kappa B kinase-dependent mechanism. Cancer Res. 2003;63:642–646. - PubMed
-
- Yoshino I, Yano T, Murata M, Ishida T, Sugimachi K, Kimura G, Nomoto K. Tumor-reactive T-cells accumulate in lung cancer tissues but fail to respond due to tumor cell-derived factor. Cancer Res. 1992;52:775–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

