cAMP signaling regulates histone H3 phosphorylation and mitotic entry through a disruption of G2 progression
- PMID: 18644368
- PMCID: PMC4416494
- DOI: 10.1016/j.yexcr.2008.06.022
cAMP signaling regulates histone H3 phosphorylation and mitotic entry through a disruption of G2 progression
Abstract
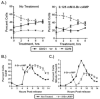
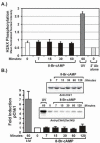
cAMP signaling is known to have significant effects on cell growth, either inhibitory or stimulatory depending on the cell type. Study of cAMP-induced growth inhibition in mammalian somatic cells has focused mainly on the combined role of protein kinase A (PKA) and mitogen-activated protein (MAP) kinases in regulation of progression through the G1 phase of the cell cycle. Here we show that cAMP signaling regulates histone H3 phosphorylation in a cell cycle-dependent fashion, increasing it in quiescent cells but dramatically reducing it in cycling cells. The latter is due to a rapid and dramatic loss of mitotic histone H3 phosphorylation caused by a disruption in G2 progression, as evidenced by the inhibition of mitotic entry and decreased activity of the CyclinB/Cdk1 kinase. The inhibition of G2 progression induced through cAMP signaling is dependent on expression of the catalytic subunit of PKA and is highly sensitive to intracellular cAMP concentration. The mechanism by which G2 progression is inhibited is independent of both DNA damage and MAP kinase signaling. Our results suggest that cAMP signaling activates a G2 checkpoint by a unique mechanism and provide new insight into normal cellular regulation of G2 progression.
Figures








Similar articles
-
Protein kinase A regulates cell cycle progression of mouse fertilized eggs by means of MPF.Dev Dyn. 2005 Jan;232(1):98-105. doi: 10.1002/dvdy.20205. Dev Dyn. 2005. PMID: 15580572
-
Inhibitory phosphorylation of a mitotic cyclin-dependent kinase regulates the morphogenesis, cell size and virulence of the smut fungus Ustilago maydis.J Cell Sci. 2005 Aug 15;118(Pt 16):3607-22. doi: 10.1242/jcs.02499. Epub 2005 Jul 26. J Cell Sci. 2005. PMID: 16046476
-
cAMP signaling induces rapid loss of histone H3 phosphorylation in mammary adenocarcinoma-derived cell lines.Exp Cell Res. 2008 Jan 1;314(1):1-10. doi: 10.1016/j.yexcr.2007.09.011. Epub 2007 Sep 22. Exp Cell Res. 2008. PMID: 17950276 Free PMC article.
-
G2 cell cycle arrest--a direct link between PKA and Cdc25C.Cell Cycle. 2003 Jan-Feb;2(1):39-41. doi: 10.4161/cc.2.1.291. Cell Cycle. 2003. PMID: 12695686 Review. No abstract available.
-
New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate.Cell Cycle. 2006 Feb;5(3):227-31. doi: 10.4161/cc.5.3.2395. Epub 2006 Feb 8. Cell Cycle. 2006. PMID: 16418576 Review.
Cited by
-
Apigenin impedes cell cycle progression at G2 phase in prostate cancer cells.Discov Oncol. 2022 Jun 7;13(1):44. doi: 10.1007/s12672-022-00505-1. Discov Oncol. 2022. PMID: 35670862 Free PMC article.
-
EPAC1 enhances brown fat growth and beige adipogenesis.Nat Cell Biol. 2024 Jan;26(1):113-123. doi: 10.1038/s41556-023-01311-9. Epub 2024 Jan 9. Nat Cell Biol. 2024. PMID: 38195707 Free PMC article.
-
PKA and PDE4D3 anchoring to AKAP9 provides distinct regulation of cAMP signals at the centrosome.J Cell Biol. 2012 Aug 20;198(4):607-21. doi: 10.1083/jcb.201201059. J Cell Biol. 2012. PMID: 22908311 Free PMC article.
References
-
- Richards JS. New signaling pathways for hormones and cyclic adenosine 3',5'-monophosphate action in endocrine cells. Mol Endocrinol. 2001;15:209–218. - PubMed
-
- Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12:258–266. - PubMed
-
- Houslay MD, Kolch W. Cell-type specific integration of cross-talk between extracellular signal-regulated kinase and cAMP signaling. Mol Pharmacol. 2000;58:659–668. - PubMed
-
- Tasken K, Stokka AJ. The molecular machinery for cAMP-dependent immunomodulation in T-cells. Biochem.Soc.Trans. 2006;34:476–479. - PubMed
-
- Koyama H, Bornfeldt KE, Fukumoto S, Nishizawa Y. Molecular pathways of cyclic nucleotide-induced inhibition of arterial smooth muscle cell proliferation. J.Cell Physiol. 2001;186:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

