Vaccination of calves using the BRSV nucleocapsid protein in a DNA prime-protein boost strategy stimulates cell-mediated immunity and protects the lungs against BRSV replication and pathology
- PMID: 18644416
- PMCID: PMC7115630
- DOI: 10.1016/j.vaccine.2008.06.100
Vaccination of calves using the BRSV nucleocapsid protein in a DNA prime-protein boost strategy stimulates cell-mediated immunity and protects the lungs against BRSV replication and pathology
Abstract
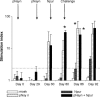
Respiratory syncytial virus (RSV) is a major cause of respiratory disease in both cattle and young children. Despite the development of vaccines against bovine (B)RSV, incomplete protection and exacerbation of subsequent RSV disease have occurred. In order to circumvent these problems, calves were vaccinated with the nucleocapsid protein, known to be a major target of CD8(+) T cells in cattle. This was performed according to a DNA prime-protein boost strategy. The results showed that DNA vaccination primed a specific T-cell-mediated response, as indicated by both a lymphoproliferative response and IFN-gamma production. These responses were enhanced after protein boost. After challenge, mock-vaccinated calves displayed gross pneumonic lesions and viral replication in the lungs. In contrast, calves vaccinated by successive administrations of plasmid DNA and protein exhibited protection against the development of pneumonic lesions and the viral replication in the BAL fluids and the lungs. The protection correlated to the cell-mediated immunity and not to the antibody response.
Figures




References
-
- Brandenburg A.H., Neijens H.J., Osterhaus A.D.M.E. Pathogenesis of RSV lower respiratory tract infection: implications for vaccine development. Vaccine. 2001;19:2769–2782. - PubMed
-
- Van der Poel W.H., Brand A., Kramps J.A., Van Oirschot T.J. Respiratory syncytial virus infections in human beings and in cattle. J Infect. 1994;29:215–228. - PubMed
-
- Belknap E.B., Baker J.C., Patterson J.S., Walker R.D., Haines D.M., Clark E.G. The role of passive immunity in bovine respiratory syncytial virus-infected calves. J Infect Dis. 1991;163:470–476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

