The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane
- PMID: 18644871
- PMCID: PMC2546938
- DOI: 10.1128/MCB.02070-07
The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane
Abstract
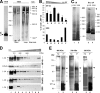
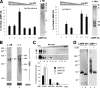
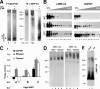
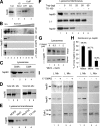
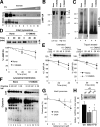
Chaperone-mediated autophagy (CMA) is a selective type of autophagy by which specific cytosolic proteins are sent to lysosomes for degradation. Substrate proteins bind to the lysosomal membrane through the lysosome-associated membrane protein type 2A (LAMP-2A), one of the three splice variants of the lamp2 gene, and this binding is limiting for their degradation via CMA. However, the mechanisms of substrate binding and uptake remain unknown. We report here that LAMP-2A organizes at the lysosomal membrane into protein complexes of different sizes. The assembly and disassembly of these complexes are a very dynamic process directly related to CMA activity. Substrate proteins only bind to monomeric LAMP-2A, while the efficient translocation of substrates requires the formation of a particular high-molecular-weight LAMP-2A complex. The two major chaperones related to CMA, hsc70 and hsp90, play critical roles in the functional dynamics of the LAMP-2A complexes at the lysosomal membrane. Thus, we have identified a novel function for hsc70 in the disassembly of LAMP-2A from these complexes, whereas the presence of lysosome-associated hsp90 is essential to preserve the stability of LAMP-2A at the lysosomal membrane.
Figures




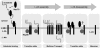
References
-
- Agarraberes, F., and J. F. Dice. 2001. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J. Cell Sci. 1142491-2499. - PubMed
-
- Aniento, F., E. Roche, A. M. Cuervo, and E. Knecht. 1993. Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J. Biol. Chem. 26810463-10470. - PubMed
-
- Auteri, J. S., A. Okada, V. Bochaki, and J. F. Dice. 1983. Regulation of intracellular protein degradation in IMR-90 human diploid fibroblasts. J. Cell. Physiol. 115167-174. - PubMed
-
- Bandhyopadhyay, U., and A. M. Cuervo. 2006. Chaperone-mediated autophagy in aging and neurodegeneration: lessons from alpha-synuclein. Exp. Gerontol. 42120-128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
