Tumor-derived extracellular mutations of PTPRT /PTPrho are defective in cell adhesion
- PMID: 18644975
- PMCID: PMC2614372
- DOI: 10.1158/1541-7786.MCR-07-2123
Tumor-derived extracellular mutations of PTPRT /PTPrho are defective in cell adhesion
Abstract
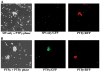
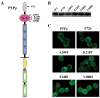
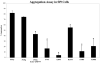
Receptor protein tyrosine phosphatase T (PTPRT/PTPrho) is frequently mutated in human cancers including colon, lung, gastric, and skin cancers. More than half of the identified tumor-derived mutations are located in the extracellular part of PTPrho. However, the functional significance of those extracellular domain mutations remains to be defined. Here we report that the extracellular domain of PTPrho mediates homophilic cell-cell aggregation. This homophilic interaction is very specific because PTPrho does not interact with its closest homologue, PTPmu, in a cell aggregation assay. We further showed that all five tumor-derived mutations located in the NH(2)-terminal MAM and immunoglobulin domains impair, to varying extents, their ability to form cell aggregates, indicating that those mutations are loss-of-function mutations. Our results suggest that PTPrho may play an important role in cell-cell adhesion and that mutational inactivation of this phosphatase could promote tumor migration and metastasis.
Figures




Similar articles
-
Cancer-derived mutations in the fibronectin III repeats of PTPRT/PTPrho inhibit cell-cell aggregation.Cell Commun Adhes. 2009 Dec;16(5-6):146-53. doi: 10.3109/15419061003653771. Cell Commun Adhes. 2009. PMID: 20230342 Free PMC article.
-
Characterization of the adhesive properties of the type IIb subfamily receptor protein tyrosine phosphatases.Cell Commun Adhes. 2010 Apr;17(2):34-47. doi: 10.3109/15419061.2010.487957. Cell Commun Adhes. 2010. PMID: 20521994 Free PMC article.
-
Synapse formation regulated by protein tyrosine phosphatase receptor T through interaction with cell adhesion molecules and Fyn.EMBO J. 2009 Nov 18;28(22):3564-78. doi: 10.1038/emboj.2009.289. Epub 2009 Oct 8. EMBO J. 2009. PMID: 19816407 Free PMC article.
-
Regulation of development and cancer by the R2B subfamily of RPTPs and the implications of proteolysis.Semin Cell Dev Biol. 2015 Jan;37:108-18. doi: 10.1016/j.semcdb.2014.09.004. Epub 2014 Sep 16. Semin Cell Dev Biol. 2015. PMID: 25223585 Free PMC article. Review.
-
Receptor protein tyrosine phosphatase micro: measuring where to stick.Biochem Soc Trans. 2008 Apr;36(Pt 2):167-72. doi: 10.1042/BST0360167. Biochem Soc Trans. 2008. PMID: 18363557 Review.
Cited by
-
Receptor-type tyrosine phosphatase ligands: looking for the needle in the haystack.FEBS J. 2013 Jan;280(2):388-400. doi: 10.1111/j.1742-4658.2012.08653.x. Epub 2012 Jul 5. FEBS J. 2013. PMID: 22682003 Free PMC article. Review.
-
Protein Tyrosine Phosphatases as Potential Regulators of STAT3 Signaling.Int J Mol Sci. 2018 Sep 11;19(9):2708. doi: 10.3390/ijms19092708. Int J Mol Sci. 2018. PMID: 30208623 Free PMC article. Review.
-
Proteolytic cleavage of protein tyrosine phosphatase mu regulates glioblastoma cell migration.Cancer Res. 2009 Sep 1;69(17):6960-8. doi: 10.1158/0008-5472.CAN-09-0863. Epub 2009 Aug 18. Cancer Res. 2009. PMID: 19690139 Free PMC article.
-
Artificial Intelligence-Based Computational Screening and Functional Assays Identify Candidate Small Molecule Antagonists of PTPmu-Dependent Adhesion.Int J Mol Sci. 2023 Feb 21;24(5):4274. doi: 10.3390/ijms24054274. Int J Mol Sci. 2023. PMID: 36901713 Free PMC article.
-
Inhibition of P53-mediated cell cycle control as the determinant in dedifferentiated liposarcomas development.Am J Cancer Res. 2021 Jun 15;11(6):3271-3284. eCollection 2021. Am J Cancer Res. 2021. PMID: 34249461 Free PMC article.
References
-
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–65. - PubMed
-
- Wang Z, Shen D, Parsons DW, et al. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science. 2004;304(5674):1164–6. - PubMed
-
- Collier LS, Carlson CM, Ravimohan S, Dupuy AJ, Largaespada DA. Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature. 2005;436(7048):272–6. - PubMed
-
- McAndrew PE, Frostholm A, White RA, Rotter A, Burghes AH. Identification and characterization of RPTP rho, a novel RPTP mu/kappa-like receptor protein tyrosine phosphatase whose expression is restricted to the central nervous system. Brain Res Mol Brain Res. 1998;56(1–2):9–21. - PubMed
-
- Ensslen-Craig SE, Brady-Kalnay SM. Receptor protein tyrosine phosphatases regulate neural development and axon guidance. Dev Biol. 2004;275(1):12–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

