eIF4E knockdown decreases breast cancer cell growth without activating Akt signaling
- PMID: 18644990
- PMCID: PMC2559956
- DOI: 10.1158/1535-7163.MCT-07-2357
eIF4E knockdown decreases breast cancer cell growth without activating Akt signaling
Abstract
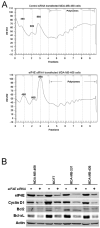
Activation of translation initiation is essential for the malignant phenotype and is emerging as a potential therapeutic target. Translation is regulated by the expression of translation initiation factor 4E (eIF4E) as well as the interaction of eIF4E with eIF4E-binding proteins (e.g., 4E-BP1). Rapamycin inhibits translation initiation by decreasing the phosphorylation of 4E-BP1, increasing eIF4E/4E-BP1 interaction. However, rapamycin also inhibits S6K phosphorylation, leading to feedback loop activation of Akt. We hypothesized that targeting eIF4E directly would inhibit breast cancer cell growth without activating Akt. We showed that eIF4E is ubiquitously expressed in breast cancer cell lines. eIF4E knockdown by small interfering RNA inhibited growth in different breast cancer cell subtypes including triple-negative (estrogen receptor/progesterone receptor/HER-2-negative) cancer cells. eIF4E knockdown inhibited the growth of cells with varying total and phosphorylated 4E-BP1 levels and inhibited rapamycin-insensitive as well as rapamycin-sensitive cell lines. eIF4E knockdown led to a decrease in expression of cyclin D1, Bcl-2, and Bcl-xL. eIF4E knockdown did not lead to Akt phosphorylation but did decrease 4E-BP1 expression. We conclude that eIF4E is a promising target for breast cancer therapy. eIF4E-targeted therapy may be efficacious in a variety of breast cancer subtypes including triple-negative tumors for which currently there are no targeted therapies. Unlike rapamycin and its analogues, eIF4E knockdown is not associated with Akt activation.
Conflict of interest statement
Figures

Similar articles
-
Mitosis-related phosphorylation of the eukaryotic translation suppressor 4E-BP1 and its interaction with eukaryotic translation initiation factor 4E (eIF4E).J Biol Chem. 2019 Aug 2;294(31):11840-11852. doi: 10.1074/jbc.RA119.008512. Epub 2019 Jun 14. J Biol Chem. 2019. PMID: 31201269 Free PMC article.
-
RhoE inhibits 4E-BP1 phosphorylation and eIF4E function impairing cap-dependent translation.J Biol Chem. 2009 Dec 18;284(51):35287-96. doi: 10.1074/jbc.M109.050120. J Biol Chem. 2009. PMID: 19850923 Free PMC article.
-
The effect of p-4E-BP1 and p-eIF4E on cell proliferation in a breast cancer model.Int J Oncol. 2011 Nov;39(5):1337-45. doi: 10.3892/ijo.2011.1118. Epub 2011 Jul 8. Int J Oncol. 2011. PMID: 21750861
-
Modeling resistance to pathway-targeted therapy in ovarian cancer.Cell Cycle. 2005 Aug;4(8):1004-6. doi: 10.4161/cc.4.8.1869. Epub 2005 Aug 25. Cell Cycle. 2005. PMID: 15970711 Free PMC article. Review.
-
Misregulation of the proline rich homeodomain (PRH/HHEX) protein in cancer cells and its consequences for tumour growth and invasion.Cell Biosci. 2016 Feb 13;6:12. doi: 10.1186/s13578-016-0077-7. eCollection 2016. Cell Biosci. 2016. PMID: 26877867 Free PMC article. Review.
Cited by
-
Knockdown of eIF4E suppresses cell growth and migration, enhances chemosensitivity and correlates with increase in Bax/Bcl-2 ratio in triple-negative breast cancer cells.Med Oncol. 2011 Dec;28(4):1302-7. doi: 10.1007/s12032-010-9630-0. Epub 2010 Jul 22. Med Oncol. 2011. PMID: 20652449
-
Structure-activity relationship study of 4EGI-1, small molecule eIF4E/eIF4G protein-protein interaction inhibitors.Eur J Med Chem. 2014 Apr 22;77:361-77. doi: 10.1016/j.ejmech.2014.03.034. Epub 2014 Mar 13. Eur J Med Chem. 2014. PMID: 24675136 Free PMC article.
-
N-Hydroxyphthalimide exhibits antitumor activity by suppressing mTOR signaling pathway in BT-20 and LoVo cells.J Exp Clin Cancer Res. 2016 Mar 3;35:41. doi: 10.1186/s13046-016-0315-1. J Exp Clin Cancer Res. 2016. PMID: 26940018 Free PMC article.
-
Reduced eIF4E function impairs B-cell leukemia without altering normal B-lymphocyte function.iScience. 2021 Jun 17;24(7):102748. doi: 10.1016/j.isci.2021.102748. eCollection 2021 Jul 23. iScience. 2021. PMID: 34278258 Free PMC article.
-
Elevated levels of p-Mnk1, p-eIF4E and p-p70S6K proteins are associated with tumor recurrence and poor prognosis in astrocytomas.J Neurooncol. 2017 Feb;131(3):485-493. doi: 10.1007/s11060-016-2327-2. Epub 2016 Nov 29. J Neurooncol. 2017. PMID: 27900644
References
-
- De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–99. - PubMed
-
- Zimmer SG, DeBenedetti A, Graff JR. Translational control of malignancy: the mRNA cap-binding protein, eIF-4E, as a central regulator of tumor formation, growth, invasion and metastasis. Anticancer Res. 2000;20:1343–51. - PubMed
-
- Rosenwald IB, Kaspar R, Rousseau D, et al. Eukaryotic translation initiation factor 4E regulates expression of cyclin D1 at transcriptional and post-transcriptional levels. J Biol Chem. 1995;270:21176–80. - PubMed
-
- Rhoads RE. Regulation of eukaryotic protein synthesis by initiation factors. J Biol Chem. 1993;268:3017–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

