Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase
- PMID: 18645010
- PMCID: PMC2562299
- DOI: 10.1158/1535-7163.MCT-08-0095
Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase
Abstract
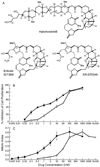
Eribulin (E7389), a synthetic analogue of halichondrin B in phase III clinical trials for breast cancer, binds to tubulin and microtubules. At low concentrations, it suppresses the growth phase of microtubule dynamic instability in interphase cells, arrests mitosis, and induces apoptosis, suggesting that suppression of spindle microtubule dynamics induces mitotic arrest. To further test this hypothesis, we measured the effects of eribulin on dynamics of centromeres and their attached kinetochore microtubules by time-lapse confocal microscopy in living mitotic U-2 OS human osteosarcoma cells. Green fluorescent protein-labeled centromere-binding protein B marked centromeres and kinetochore-microtubule plus-ends. In control cells, sister chromatid centromere pairs alternated under tension between increasing and decreasing separation (stretching and relaxing). Eribulin suppressed centromere dynamics at concentrations that arrest mitosis. At 60 nmol/L eribulin (2 x mitotic IC(50)), the relaxation rate was suppressed 21%, the time spent paused increased 67%, and dynamicity decreased 35% (but without reduction in mean centromere separation), indicating that eribulin decreased normal microtubule-dependent spindle tension at the kinetochores, preventing the signal for mitotic checkpoint passage. We also examined a more potent, but in tumors less efficacious antiproliferative halichondrin derivative, ER-076349. At 2 x IC(50) (4 nmol/L), mitotic arrest also occurred in concert with suppressed centromere dynamics. Although media IC(50) values differed 15-fold between the two compounds, the intracellular concentrations were similar, indicating more extensive relative uptake of ER-076349 into cells compared with eribulin. The strong correlation between suppression of kinetochore-microtubule dynamics and mitotic arrest indicates that the primary mechanism by which eribulin blocks mitosis is suppression of spindle microtubule dynamics.
Conflict of interest statement
Conflicts of Interest: Drs. Okouneva, Azarenko, Wilson, and Jordan received or were supported in part by a research grant from Eisai Research Institute. Dr. Littlefield is an employee of Eisai Research Institute.
Figures




References
-
- Jordan MA, Kamath K, Manna T, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4:1086–1095. - PubMed
-
- Dabydeen D, Burnett J, Bai R, et al. Comparison of the activities of the truncated halichondrin B analog NSC 707389 (E7389) with those of the parent compound and a proposed binding site on tubulin. Mol Pharmacol. 2006;70:1866–1875. - PubMed
-
- Kuznetsov G, Towle MJ, Cheng H, et al. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res. 2004;64:5760–5766. - PubMed
-
- Towle MJ, Salvato KA, Budrow J, et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogs of halichondrin B. Cancer Res. 2001;61:1013–1021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

