Intermediate acting versus long acting insulin for type 1 diabetes mellitus
- PMID: 18646147
- PMCID: PMC6486116
- DOI: 10.1002/14651858.CD006297.pub2
Intermediate acting versus long acting insulin for type 1 diabetes mellitus
Abstract
Background: Diabetes mellitus type 1 is a chronic disease with short and long term complications. Its goals of therapy are to eliminate the symptoms of hyperglycaemia, reduce the long term microvascular and macrovascular complications and allow the patients to achieve a normal life-style. Basal insulin replacement for insulin dependent patients can be achieved with either intermediate or long acting insulin preparations.
Objectives: To assess the effects of intermediate acting versus long acting insulin preparations for basal insulin replacement in type 1 diabetic patients.
Search strategy: We searched MEDLINE, EMBASE and The Cochrane Library, as well as reference lists, databases of ongoing trials, and requests from authors of included trials.
Selection criteria: Randomised controlled trials, assessing long acting insulin preparations compared to intermediate acting insulin preparations, in type 1 diabetic patients.
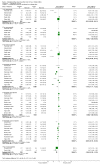
Data collection and analysis: Two reviewers independently scanned the titles. Data were extracted and analysed accordingly.
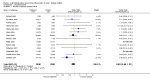
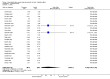
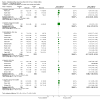
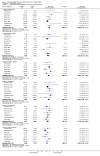
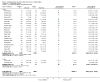
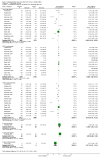
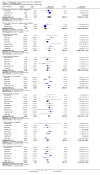
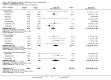
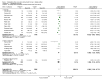
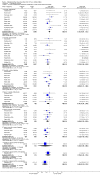
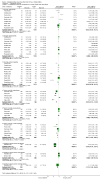
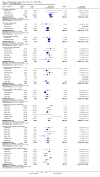
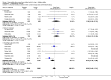
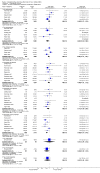
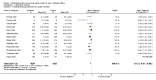
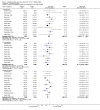
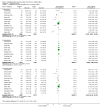
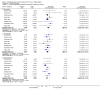
Main results: Twenty-three randomised controlled trials were identified. A total of 3872 and 2915 participants in the intervention and in the control group, respectively, were analysed. The weighted mean difference (WMD) for the level of glycosylated haemoglobin was -0.08 (95% confidence interval (CI) -0.12 to -0.04) in favour of the long acting insulin arm. The WMD between the groups in fasting plasma and blood glucose levels was -0.63 (95% CI -0.86 to -0.40) and -0.86 (95% CI -1.00 to -0.72) in favour of the long acting insulins. The odds ratio for a patient on long acting insulin to develop any type of hypoglycaemia was 0.93 (95% CI 0.8 to 1.08) compared to that of a patient on intermediate acting insulins. The OR for severe hypoglycaemic episodes was 0.73 (95% CI 0.61 to 0.87), and 0.70 (95% CI of 0.63 to 0.79) for nocturnal episodes. The WMD between the long and intermediate insulin groups for hypoglycaemic events per 100 patient follow up days was -0.77 (95% CI -0.89 to -0.65), -0.0 (95% CI -0.02 to 0.02) and -0.40 (95% CI -0.45 to -0.34) for overall, severe, and nocturnal hypoglycaemic episodes. Weight gain was more prominent in the control group. No difference was noted in the quantity or quality of severe adverse events or deaths.
Authors' conclusions: Long acting insulin preparations seem to exert a beneficial effect on nocturnal glucose levels. Their effect on the overall diabetes control is clinically unremarkable. Their use as a basal insulin regimen for type 1 diabetes mellitus warrants further substantiation.
Conflict of interest statement
None known.
Figures
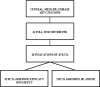
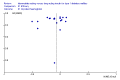
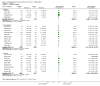







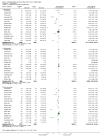


























































Update of
References
References to studies included in this review
Ashwell 2006 {published data only}
-
- Ashwell SG, et al. Improved glycaemic control with insulin glargine plus insulin lispro: a multicentre, randomized, cross‐over trial in people with Type 1 diabetes. Diabetic Medicine 2006;23(3):285‐92. - PubMed
Chatterjee 2007 {published data only}
-
- Chatterjee S, Jarvis‐Kay J, Rengarajan T, Lawrence IG, McNally PG, Davies MJ. Glargine versus NPH insulin: Efficacy in comparison with insulin aspart in a basal bolus regimen in type 1 diabetes—The glargine and aspart study (GLASS). A randomised cross‐over study. Diabetes Research and Clinical Practice 2006;77:215‐22. - PubMed
De Leeuw 2005 {published data only}
-
- De Leeuw, et al. Insulin detemir used in basal‐bolus therapy in people with type 1 diabetes is associated with a lower risk of nocturnal hypoglycaemia and less weight gain over 12 months in comparison to NPH insulin. Diabetes, Obesity & Metabolism 2005;7(1):73‐82. - PubMed
Francis 1986 {published data only}
-
- Francis AJ, Hanning I, Alberti KGMM. Human ultralente insulin: A comparison with porcine lente insulin as a twice‐daily insulin in insulin‐dependent diabetic patients with fasting hyperglycaemia. Diabetes Research 1986;3(5):263‐8. - PubMed
Fulcher 2005 {published data only}
-
- Fulcher GR, Gilbert RE, Yue DK. Glargine is superior to neutral protamine Hagedorn for improving glycated haemoglobin and fasting blood glucose levels during intensive insulin therapy. Internal Medicine Journal 2005;35(9):536‐42. - PubMed
Hermansen 2001 {published data only}
-
- Hermansen K, et al. Comparison of the soluble basal insulin analog insulin detemir with NPH insulin: a randomized open crossover trial in type 1 diabetic subjects on basal‐bolus therapy. Diabetes Care 24;2:296‐301. - PubMed
Hermansen 2004 {published data only}
-
- Hermansen K, et al. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal‐bolus therapy for patients with type 1 diabetes. Diabetologia 2004;47(4):622‐9. - PubMed
Home 2004 {published data only}
-
- Home P, et al. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes: a randomized clinical trial. Diabetes Care 2004;27(5):1081‐7. - PubMed
Home 2005 {published data only}
-
- Home PD, et al. A randomized multicentre trial of insulin glargine compared with NPH insulin in people with type 1 diabetes. Diabetes/Metabolism Research Reviews 2005;21(6):545‐53. - PubMed
-
- Witthaus E, Stewart J, Bradley C. Treatment satisfaction and psychological well‐being with insulin glargine compared with NPH in patients with Type 1 diabetes. Diabetic Medicine 2001;18:619‐25. - PubMed
Kolendorf 2006 {published data only}
-
- Kolendorf K, et al. Insulin detemir lowers the risk of hypoglycaemia and provides more consistent plasma glucose levels compared with NPH insulin in Type 1 diabetes. Diabetic Medicine 2006;23(7):729‐35. - PubMed
Murphy 2003 {published data only}
-
- Murphy NP, et al. Randomized cross‐over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care 2003;26(3):799‐804. - PubMed
Pieber 2000 {published data only}
-
- Pieber TR, Eugene‐Jolchine I, Derobert E. Efficacy and safety of HOE 901 versus NPH insulin in patients with type 1 diabetes. The European Study Group of HOE 901 in type 1 diabetes. Diabetes Care 2000;23(2):157‐62. - PubMed
Porcellati 2004 {published data only}
-
- Porcellati F, et al. Better long‐term glycaemic control with the basal insulin glargine as compared with NPH in patients with Type 1 diabetes mellitus given meal‐time lispro insulin. Diabetic Medicine 2004;21(11):1213‐20. - PubMed
Raskin 2000 {published data only}
-
- Raskin P, et al. A 16‐week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care 2000;23(11):1666‐71. - PubMed
Ratner 2000 {published data only}
-
- Ratner RE, et al. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care 2000;23(5):639‐43. - PubMed
Robertson 2007 {published data only}
-
- Robertson KJ, Schoenle E, Gucev Z, Mordhorst L, Gall MA, Ludvigsson J. Insulin detemir compared with NPH insulin in children and adolescents with Type 1 diabetes. Diabetic medicine 2007;24:27‐34. - PubMed
Rosenstock 2000 {published data only}
-
- Rosenstock J, et al. Basal insulin glargine (HOE 901) versus NPH insulin in patients with type 1 diabetes on multiple daily insulin regimens. U.S. Insulin Glargine (HOE 901) Type 1 Diabetes Investigator Group. Diabetes Care 2000;23(8):1137‐42. - PubMed
Rossetti 2003 {published data only}
-
- Rossetti P, Pampanelli S, Fanelli C, Porcellati F, Costa E, Torlone E, et al. Intensive replacement of basal insulin in patients with type 1 diabetes given rapid‐acting insulin analog at mealtime: a 3‐month comparison between administration of NPH insulin four times daily and glargine insulin at dinner or bedtime. Diabetes Care 2003;26(5):1490‐6. [Intensive replacement of basal insulin in patients with type 1 diabetes given rapid‐acting insulin analog at mealtime: a 3‐month comparison between administration of NPH insulin four times daily and glargine insulin at dinner or bedtime. Diabetes Care. 2003 May;26(5):1490‐6. Rossetti P, Pampanelli S, Fanelli C, Porcellati F, Costa E, Torlone E, Scionti L, Bolli GB. Related Articles, Links Intensive replacement of basal insulin in patients with type 1 diabetes given rapid‐acting insulin analog at mealtime: a 3‐month comparison between administration of NPH insulin four times daily and glargine insulin at dinner or bedtime. Diabetes Care. 2003 May;26(5):1490‐6.] - PubMed
Russell‐Jones 2004 {published data only}
-
- Russell‐Jones D, et al. Effects of QD insulin detemir or neutral protamine Hagedorn on blood glucose control in patients with type I diabetes mellitus using a basal‐bolus regimen. Clinical Therapeutics 2004;26(5):724‐36. - PubMed
Schober 2001 {published data only}
-
- Schober E, et al. Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes. Diabetes Care 2001;24(11):2005‐6. - PubMed
Tunbridge 1989 {published data only}
-
- Tunbridge FK, et al. A comparison of human ultralente‐ and lente‐based twice‐daily injection regimens. Diabetic Medicine 1989;6(6):496‐501. - PubMed
Vague 2003 {published data only}
-
- Vague P, Selam JL, Skeie S, Leeuw I, Elte JW, Haahr H, Kristensen A, Draeger E. Insulin detemir is associated with more predictable glycemic control and reduced risk of hypoglycemia than NPH insulin in patients with type 1 diabetes on a basal‐bolus regimen with premeal insulin aspart. Diabetes Care 2003;26(3):590‐6. - PubMed
Zinman 1999 {published data only}
-
- Zinman B, et al. Effectiveness of human ultralente versus NPH insulin in providing basal insulin replacement for an insulin lispro multiple daily injection regimen. A double‐blind randomized prospective trial. The Canadian Lispro Study Group. Diabetes Care 1999;22(4):603‐8. - PubMed
References to studies excluded from this review
Albright 2004 {published data only}
-
- Albright ES, Desmond R, Bell DS. Efficacy of conversion from bedtime NPH insulin injection to once‐ or twice‐daily injections of insulin glargine in type 1 diabetic patients using basal/bolus therapy. Diabetes Care 2004;27(2):632‐3. - PubMed
Alemzadeh 2003 {published data only}
-
- Alemzadeh R, et al. Beneficial effects of flexible insulin therapy in children and adolescents with type 1 diabetes mellitus. Acta Diabetologica 40;3:137‐42. - PubMed
Alemzadeh 2005 {published data only}
-
- Alemzadeh R, Berhe T, Wyatt DT. Flexible insulin therapy with glargine insulin improved glycemic control and reduced severe hypoglycemia among preschool‐aged children with type 1 diabetes mellitus. Pediatrics 2005;115(5):1320‐4. - PubMed
Heise 2003 {published data only}
-
- Heise T, et al. Lower Within‐Subject Variability of Insulin Detemir in Comparison to NPH Insulin and Insulin Glargine in People With Type 1 Diabetes. Diaetes 2003;53:1614‐20. - PubMed
Hershon 2004 {published data only}
-
- Hershon KS, et al. Once‐daily insulin glargine compared with twice‐daily NPH insulin in patients with type 1 diabetes. Endocrine Practice 2004;10(1):10‐7. - PubMed
Herwig 2007 {published data only}
-
- Herwig J, Scholl‐Schilling G, Bohles H. Glycaemic control and hypoglycaemia in children, adolescents and young adults with unstable type 1 diabetes mellitus treated with insulin glargine or intermediate‐acting insulin. Jounal of pediatric endocrinology and metabolism 2007;20(4):517‐25. - PubMed
Kiess 2004 {published data only}
-
- Kiess W, et al. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes. Diabetes Care 2004;27(10):2567‐8. - PubMed
Lepore 2000 {published data only}
-
- Lepore M, Pampanelli S, Fanelli C, Porcellati F, Bartocci L, Di VA, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long‐acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000;49(12):2142‐8. - PubMed
Lepore 2003 {published data only}
-
- Lepore G, et al. Both continuous subcutaneous insulin infusion and a multiple daily insulin injection regimen with glargine as basal insulin are equally better than traditional multiple daily insulin injection treatment. Diabetes Care 2003;26(4):1321‐2. - PubMed
Manini 2007 {published data only}
-
- Manini R, Forlani G, Moscatiello S, Zannoni C, Marzocchi R, Marchesini G. Insulin glargine improves glycemic control and health‐related quality of life in type 1 diabetes. Nutrition, Metabolism & Cardiovascular Diseases 2007;17:493‐8. - PubMed
Mimouni 1979 {published data only}
-
- Mimouni M, et al. Comparison of one daily injection of NPH and crystalline PZI insulins on urinary glucose in diabetic children. Acta Diabetologica Latina 1979;16(1):63‐6. - PubMed
Moretti 2005 {published data only}
-
- Moretti V, et al. Treatment with glargine in adolescents with type 1 diabetes mellitus: Clinical and metabolic control after 9 months. Rivista Italiana di Medicina Dell'Adolescenza 2005;3(1):36‐41.
Palmer 2004 {published data only}
-
- Palmer AJ, et al. Cost‐effectiveness of detemir‐based basal/bolus therapy versus NPH‐based basal/bolus therapy for type 1 diabetes in a UK setting: an economic analysis based on meta‐analysis results of four clinical trials. Current Medical Research & Opinion 2004;20(11):1729‐46. - PubMed
Ratner 2003 {published data only}
-
- Ratner R. Insulin glargine versus NPH insulin in patients with type 1 diabetes. Drugs of Today 2003;39(11):867‐76. - PubMed
Saisho 2005 {published data only}
-
- Saisho Y, et al. Effect of switching from NPH insulin to insulin glargine on glycemic control in Japanese type 1 diabetic patients. Journal of the Japan Diabetes Society 2005;48(9):685‐91.
Urakami 2007 {published data only}
-
- Urakami T, et al. Usefulness of long acting insulin analogue glargine in basal‐bolus therapy for Japanese children and adolscents with type 1 diabetes mellitus. Journal of Pediatric endocrinology and metabolism 2007;20:807‐15. - PubMed
Wutte 2007 {published data only}
-
- Wutte A, et al. Proportional dose‐response relationship and lower within‐patient variability of insulin detemir and NPH insulin in subjects with type 1 diabetes mellitus. Exp Clin Endocrinol Diabetes 2007;115(7):461‐7. - PubMed
References to ongoing studies
Alcolado 2001 {unpublished data only}
-
- Efficacy and safety of basal insulin analogue detemir and NPH insulin in patients with Type 1 diabetes on basal‐bolus regimen. Ongoing study 1.2.01.
Page 2001 {published data only}
-
- Page M. 16 week multi‐center, open, randomised 3 group parallel study comparing insuln determir with NPH. The National Research Register (NRR) Archive.
Additional references
ADA 1997
-
- American Diabetes Association. Report on the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20 Suppl 1:S5‐20. - PubMed
ADA 1999
-
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22 Suppl 1:S5‐19. - PubMed
Bolli 1999
-
- Bolli GB, Marchi RD, Park GD, Pramming S, Koivisto VA. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia 1999;42:1151‐67. - PubMed
Cohen 1960
-
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46.
Havelund 2004
-
- Havelund S, Plum A, Ribel U, Jonassen I, Volund A, Markussen J, et al. The mechanism of protraction of insulin detemir, a long‐acting, acylated analog of human insulin. Pharmaceutical research 2004;21(8):1498‐504. - PubMed
Heinemann 2000
-
- Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time‐action profile of the long‐acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 2000;23(5):644‐9. - PubMed
Heise 2004
-
- Heise T, Nosek L, Ronn BB, Endahl L, Heinemann L, Kapitza C, et al. Lower within‐subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004;53(6):1614‐20. - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. - PubMed
Higgins 2003
Higgins 2005
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, Ltd.
Hirsch 1998
-
- Hirsch IB. Intensive treatment of type 1 diabetes. The Medical clinics of North America 1998;82:689‐719. - PubMed
Horvath 2007
-
- Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, Kaiser T, Pieber TR, Siebenhofer A. Long‐acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005613.pub3] - DOI - PubMed
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Kurtzhals 2000
-
- Kurtzhals P, Schaffer L, Sorensen A, Kristensen C, Jonassen I, Schmid C, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use.. Diabetes 2000;49(6):999‐1005. - PubMed
Moher 1999
-
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. - PubMed
Rizza 1986
-
- Rizza R, O'Brien P, Service F. Use of beef ultralente for basal insulin delivery: plasma insulin concentrations after chronic ultralente administration in patients with IDDM. Diabetes Care 1986;9:120‐3. - PubMed
Rosenstock 2005
-
- Rosenstock J, Dailey G, Massi‐Benedetti M, Fritsche A, Lin Z, Salzman A. Reduced hypoglycemia risk with Insulin Glargine: A meta‐analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care 2005;28:950‐5. - PubMed
Rosskamp 1999
-
- Rosskamp R, Park G. Long acting insulin analogues. Diabetes Care 1999;22 (Suppl 2):B109‐13. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;237:408‐12. - PubMed
WHO 1980
-
- WHO Expert Committee on Diabetes Mellitus. Second report. Technical Report Series 646. Geneva. WHO, 1980. - PubMed
WHO 1985
-
- WHO Expert Committee on Diabetes Mellitus. World Health Organization, 1985. Technical Report Series 727. - PubMed
WHO 1998
-
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its compliactions. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. - PubMed
Witthaus 2001
-
- Witthaus E, Stewart J, Bradley C. Treatment satisfaction and psychological well‐being with insulin glargine compared with NPH in patients with Type 1 diabetes. Diabetic Medicine 2001;18:619‐25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

