Regular treatment with salmeterol for chronic asthma: serious adverse events
- PMID: 18646149
- PMCID: PMC4015854
- DOI: 10.1002/14651858.CD006363.pub2
Regular treatment with salmeterol for chronic asthma: serious adverse events
Abstract
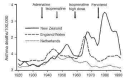
Background: Epidemiological evidence has suggested a link between beta-agonists and increases in asthma mortality. There has been much debate about possible causal links for this association, and whether regular (daily) long-acting beta(2)-agonists are safe.
Objectives: The aim of this review is to assess the risk of fatal and non-fatal serious adverse events in trials that randomised patients with chronic asthma to regular salmeterol versus placebo or regular short-acting beta(2)-agonists.
Search strategy: Trials were identified using the Cochrane Airways Group Specialised Register of trials. Web sites of clinical trial registers were checked for unpublished trial data and FDA submissions in relation to salmeterol were also checked. The date of the most recent search was October 2007.
Selection criteria: Controlled parallel design clinical trials on patients of any age and severity of asthma were included if they randomised patients to treatment with regular salmeterol and were of at least 12 weeks duration. Concomitant use of inhaled corticosteroids was allowed, as long as this was not part of the randomised treatment regimen.
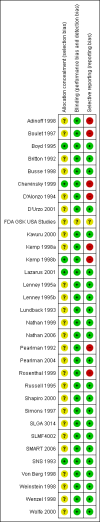
Data collection and analysis: Two authors independently selected trials for inclusion in the review. Outcome data was extracted by one author and checked by the second author. Unpublished data on mortality and serious adverse events was sought.
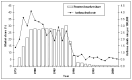
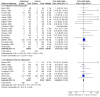
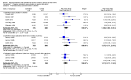
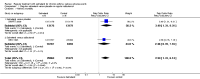
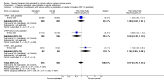
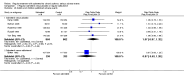
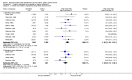
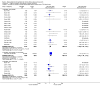
Main results: The review includes 26 trials comparing salmeterol to placebo and 8 trials comparing with salbutamol. These included 62,630 participants with asthma (including 2,380 children). In 6 trials (2,766 patients), no serious adverse event data could be obtained. All cause mortality was higher with regular salmeterol than placebo but the increase was not significant, Odds Ratio 1.33 [95% CI: 0.85, 2.10]. Non-fatal serious adverse events were significantly increased when regular salmeterol was compared with placebo, Odds Ratio 1.14 [95% CI: 1.01, 1.28]. One extra serious adverse event occurred over 28 weeks for every 188 people treated with regular salmeterol [95% CI: 95 to 2606]. There is insufficient evidence to assess whether the risk in children is higher or lower than in adults. No significant increase in fatal or non-fatal serious adverse events was found when regular salmeterol was compared with regular salbutamol. Individual patient data from the SNS study have been combined with the results of the SMART study; in patients who were not taking inhaled corticosteroids, compared to regular salbutamol or placebo, there was a significant increase in risk of asthma-related death with regular salmeterol, Odds Ratio 9.52 [95% CI: 1.24, 73.09]. The confidence interval for patients taking inhaled corticosteroids is too wide to rule out an increase in asthma mortality in this group.
Authors' conclusions: In comparison with placebo, we have found an increased risk of serious adverse events with regular salmeterol. There is also a clear increase in risk of asthma-related mortality in patients not using inhaled corticosteroids in the two large surveillance studies. Although the increase in asthma-related mortality was smaller in patients taking inhaled corticosteroids at baseline, the confidence interval is wide, so it cannot be concluded that the inhaled corticosteroids abolish the risks of regular salmeterol. The adverse effects of regular salmeterol in children remain uncertain due to the small number of children studied.
Conflict of interest statement
None known.
Figures


























Comment in
-
Review: daily salmeterol increases non-fatal serious adverse events in chronic asthma.Evid Based Med. 2009 Feb;14(1):14. doi: 10.1136/ebm.14.1.14. Evid Based Med. 2009. PMID: 19181946 No abstract available.
-
Should salmeterol be used for long-term asthma control?Am Fam Physician. 2009 Jun 1;79(11):957-8; quiz 935-7. Am Fam Physician. 2009. PMID: 19514693 Review. No abstract available.
References
References to studies included in this review
Adinoff 1998 {published data only}
-
- Adinoff A, Schwartz H, Rickard K, Yancey S, Swearingen B. Salmeterol compared with current therapies in chronic asthma. Journal of Family Practice 1998;47(4):278‐84. - PubMed
Boulet 1997 {published data only}
-
- Boulet LP, Laviolette M, Boucher S, Knight A, Hébert J, Chapman KR. A twelve week comparison of salmeterol and salbutamol in the treatment of mild‐to‐moderate asthma: a Canadian multicentre study. Journal of Allergy and Clinical Immunology 1997;99(1 Pt 1):13‐21. - PubMed
Boyd 1995 {published data only}
-
- Boyd G. Salmeterol xinafoate in asthmatic patients under consideration for maintenance oral corticosteroid therapy. European Respiratory Journal 1995;8:1494‐98. - PubMed
Britton 1992 {published data only}
-
- Britton MG, Earnshaw JS, Palmer JBD. A twelve month comparison of salmeterol with salbutamol in asthmatic patients. European Respiratory Journal 1992;5(9):1062‐7. - PubMed
-
- SLGT02. Inhaled GR33343G In Reversible Airways Obstruction – Efficacy Over 3 Months and Safety Over 12 Months. http://ctr.gsk.co.uk/Summary/salmeterol/III_SLGT02.pdf.
Busse 1998 {published data only}
-
- Busse WW, Casale TB, Murray JJ, Petrocella V, Cox F, Rickard K. Efficacy, safety, and impact on quality of life of salmeterol in patients with moderate persistent asthma. American Journal of Managed Care 1998;4(11):1579‐87. - PubMed
Chervinsky 1999 {published data only}
-
- Chervinsky P, Goldberg P, Galant S, Wang Y, Arledge T, Welch MB, et al. Long‐term cardiovascular safety of salmeterol powder pharmacotherapy in adolescent and adult patients with chronic persistent asthma: a randomized clinical trial. Chest 1999;115(3):642‐8. - PubMed
D'Alonzo 1994 {published data only}
-
- D'Alonzo GE. Efficacy of inhaled salmeterol in the treatment of asthma. European Respiratory Review 1995;5:128‐32.
-
- D'Alonzo GE, Nathan RA, Henchowicz MD. Salmeterol xinafoate as maintenance therapy compared with albuterol in patients with asthma. JAMA 1994;271(18):1412‐6. - PubMed
D'Urzo 2001 {published data only}
-
- D'Urzo AD, Chapman KR, Cartier A, Hargreave FE, Fitzgerald M, Tesarowski D. Effectiveness and safety of salmeterol in non‐specialist practice settings. Chest 2001;119(3):714‐9. - PubMed
-
- SLGQ94 (521/180). A multicenter, randomized, double‐blind, parallel‐group trial to evaluate the long‐term efficacy and safety of inhaled salmeterol 50ìg BID compared to short‐acting â2‐agonists as‐needed in adult patients with asthma. http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
FDA GSK USA Studies {published data only}
-
- GlaxoSmithKline. Safety of Long‐Acting Beta‐Agonist Bronchodilators. http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005‐4148B1_01_01‐GSK‐Se... 2005.
Kavuru 2000 {published and unpublished data}
-
- Edwards T, Gross G, Mitchell D, Chervinsky P, Woodring A, Baitinger L, et al. The salmeterol xinafoate/fluticasone propionate dry powder combination product via diskus(r) inhaler improves asthma control compared to salmeterol xinafoate or fluticasone propionate dry powder alone. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A414.
-
- Kavuru M, Melamed J, Gross G, Laforce C, House K, Prillaman B, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomized, double‐blind, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 2000;106(6 (pt 1)):1108‐16. - PubMed
-
- Nathan R, Woodring A, Baitinger L, Prillaman B, Faris M, House K, et al. The salmeterol/fluticasone propionate Diskus combination decreases the incidence of exacerbations compared to treatment with salmeterol or fluticasone propionate alone. European Respiratory Journal 1999;14:123S.
-
- SFCA3002. A randomized, double‐blind, parallel‐group trial evaluating safety and efficacy of salmeterol 50mcg BID and fluticasone propionate 100mcg BID individually and in combination and placebo in subjects with asthma. http://ctr.gsk.co.uk 2005.
Kemp 1998a {published data only}
-
- Kemp J, Wolfe J, Grady J, LaForce C, Stahl E, Arlidge T, et al. Salmeterol powder compared with albuterol aerosol as maintenance therapy for asthma in adolescent and adult patients. Clinical Therapeutics 1998;20(2):270‐82. - PubMed
-
- Wolfe J, LaForce C, Chervinsky P, Galant S, Lumry W, Noonan M, et al. Inhaled salmeterol powder compared with albuterol aerosol given regularly or as needed for asthma. Annals of Allergy, Asthma & Immunology 1995;74:52.
Kemp 1998b {published data only}
-
- Kemp JP, Cook DA, Incaudo GA, Corren J, Kalberg C, Emmett A, et al. Salmeterol improves quality of life in patients with asthma requiring inhaled corticosteroids. Journal of Allergy and Clinical Immunology 1998;101:188‐95. - PubMed
Lazarus 2001 {published data only}
-
- Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, et al. Long‐acting {beta}2‐agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285(20):2583‐93. - PubMed
Lenney 1995a {published data only}
-
- Lenney W, Pedersen S, Boner AL, Ebbutt A, Jenkins MM. Efficacy and safety of salmeterol in childhood asthma. European Journal of Pediatrics 1995;154:983‐90. - PubMed
-
- SLPT01. A Multicentre, Randomized, Double‐Blind, 3‐Limbed, Parallel‐Group Study Comparing the Efficacy and Tolerability of Salmeterol Xinafoate 25ìg and 50ìg BID With Salbutamol 200ìg BID Given via Pressurized Inhalers in Children With Reversible Airways Obstruction. (3‐Month Efficacy and Safety Study.). http://ctr.gsk.co.uk/Summary/salmeterol/III_SLPT01.pdf.
-
- SMS40093 (SLPT01). A multi‐centre, randomised, double blind, three limbed, parallel‐group study comparing the efficacy and tolerability of salmeterol xinafoate 25ìg and 50ìg bd with salbutamol 200ìg bd given via pressurised inhalers in children with reversible airways obstruction. (Results of the final nine months safety phase.). http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
Lenney 1995b {published data only}
-
- Lenney W, Pedersen S, Boner AL, Ebbutt A, Jenkins MM. Efficacy and safety of salmeterol in childhood asthma. European Journal of Pediatrics 1995;154:983‐90. - PubMed
-
- SLPT02. A Multi‐centre, Randomised, Double‐Blind, 3‐Limbed, Parallel‐Group Study Comparing the Efficacy and Tolerability of Dry Powder Formulations of Salmeterol Xinafoate 25ìg and 50ìg Twice Daily With Salbutamol 200ìg Twice Daily Given in Children With Reversible Airways Obstruction. http://ctr.gsk.co.uk/Summary/salmeterol/III_SLPT02.pdf.
Lundback 1993 {published data only}
-
- SLGT06. Inhaled GR33343G in Reversible Airways Obstruction – Efficacy and Safety Over Three Months: A Double‐Blind, Parallel‐Group Study Comparing Dry Powder Formulations of Inhaled GR33343G (50mcg) Administered Twice a Day and Inhaled Salbutamol (400mcg) Administered Four Times a Day. http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
Nathan 1999 {published data only}
-
- Nathan R, Pinnas J, Schwartz H, Grossman J, Yancey S, Emmett A, et al. A six‐month, placebo‐controlled comparison of the safety and efficacy of salmeterol or beclomethasone for persistent asthma. Annals of Allergy 1999;82(6):521‐9. - PubMed
Nathan 2006 {published and unpublished data}
-
- Edin HM, Payne E, Herrle MR, Schoaf L, Mather DB, Scott CA, et al. Salmeterol/fluticasone propionate combination via HFA MDI improves quality of life. Journal of Allergy and Clinical Immunology 2001;107(2):S246.
-
- Nathan RA, Mitchell D, Condemi J, Heller A, Schoaf L, Herrle M, et al. Cardiovascular and hypothalmic‐pituitary‐adrenal axis safety of fluticasone propionate/salmeterol HFA MDI in adolescent and adult patients with asthma. American Journal for Respiratory and Critical Care Medicine 2001;163(5):A863.
-
- Nathan RA, Rooklin A, Schoaf L, Scott C, Ellsworth A, House K, et al. Efficacy and tolerability of fluticasone propionate/salmeterol administered twice daily via hydrofluoroalkane 134a metered‐dose inhaler in adolescent and adult patients with persistent asthma: a randomized, double‐blind, placebo‐controlled, 12‐week study. Clinical Therapeutics 2006;28(1):73‐85. - PubMed
-
- Pearlman DS, Kent E, Lanz MJ, Peden D, Baitinger L, Herrle M, et al. Fluticasone propionate/salmeterol HFA MDI has a rapid onset of effect in asthmatics treated with short‐ or long‐acting beta‐agonists (BA) or inhaled corticosteroids (ICS). Amercian Journal of Respiratory and Critical Care Medicine 2001;163(5):A865.
-
- Rooklin A, Elkayam D, Weiler J, Windom H, Schoaf L, Scott C, et al. The fluticasone propionate/salmeterol HFA MDI is significantly more efficacious in treating asthma than placebo HFA MDI, fluticasone propionate CFC MDI or salmeterol CFC MDI. Journal of Allergy and Clinicial Immunology 2001;107(2):100s.
Pearlman 1992 {published data only}
-
- Arledge T, Liddle D, Stahl E, Rossing T. Salmeterol does not cause tolerance during long term asthma therapy. Journal of Allergy and Clinical Immunology 1996;98(6 Pt 1):1116‐9. - PubMed
-
- Nathan R, Seltzer J, Kemp J, Chervinsky P, Alexander W, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma & Immunology 1995;75(3):243‐8. - PubMed
-
- Pearlman DS. Long‐acting beta2‐agonist salmeterol compared with albuterol in maintenance asthma therapy. Annals of Allergy, Asthma & Immunology 1995;75(2):180‐4. - PubMed
-
- Pearlman DS, Chervinsky P, LaForce C, Seltzer JM, Southern DL, Kemp JP, et al. A comparison of salmeterol with albuterol in the treatment of mild‐to‐moderate asthma. New England Journal of Medicine 1992;327(20):1420‐5. - PubMed
-
- Pearlman DS, Liddle R. Controlling asthma symptoms: salmeterol compared with salbutamol in large‐scale multicentre studies. European Respiratory Review 1994;4(21):301‐5.
Pearlman 2004 {published and unpublished data}
-
- Pearlman DS, Peden D, Condemi JJ, Weinstein S, White M, Baitinger L, et al. Efficacy and safety of fluticasone propionate/salmeterol HFA 134A MDI in patients with mild‐to‐moderate persistent asthma. Journal of Asthma 2004;41(8):797‐806. - PubMed
-
- SAS30003. A stratified, randomised, double‐blind, placebo‐controlled, parallel‐group, 12‐week trial evaluating the safety and efficacy of the salmeterol/fluticasone propionate combination in HFA 134a MDI, 42/88 mcg BID, and salmeterol in propellant 11/12 MDI, 42 mcg BID, fluticasone propionate in propellant 11/12 MDI, 88 mcg BID, and placebo propellant HFA 134a MDI in adult and adolescent subjects with asthma. http://ctr.gsk.co.uk 2005.
-
- Weinstein SF, Pearlman DS, Condemi JJ, Herrle MR, Scott CA, Payne JE, et al. Superior efficacy of the fluticasone propionate/salmeterol 88/42 mcg HFA‐MDI combination product versus the individual components in asthmatics previously treated with either short‐ or long‐acting beta2‐agonists or inhaled corticosteroids. Journal of Allergy & Clinial Immunology 2001;107(2):S102.
Rosenthal 1999 {published data only}
-
- Rosenthal R, Busse W, Kemp J, Baker J, Kalberg C, Emmett A, et al. Effect of long‐term salmeterol therapy compared with as‐needed albuterol use on airway hyperresponsiveness. Chest 1999;116(3):595‐602. - PubMed
Russell 1995 {published data only}
-
- Russell G, Williams DA, Weller P, Price JF. Salmeterol xinafoate in children on high dose inhaled steroids. Annals of Allergy, Asthma & Immunology 1995;75(5):423‐8. - PubMed
-
- SALMP/AH91/D89. A phase III, multi‐centre, double‐blind, placebo controlled, parallel group study assessing the efficacy and safety of inhaled salmeterol xinafoate (Serevent™) 50 micrograms BD via the Diskhaler™ when added to the existing treatment of moderate to severe asthmatic children.. http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
Shapiro 2000 {published data only}
-
- SFCA3003. A randomized, double‐blind, parallel‐group trial evaluating safety and efficacy of salmeterol 50 mcg BID and fluticasone propionate 250 mcg BID individually and in combination and placebo in subjects with asthma. http://ctr.gsk.co.uk/Summary/fluticasone_salmeterol/III_SFCA3003.pdf 2005.
-
- Shapiro G, Lumry W, Wolfe J, Given J, White M, Woodring A, et al. Combined salmeterol 50 mcg and fluticasone propionate 250 mcg in the Diskus device for the treatment of asthma. American Journal of Respiratory and Critical Care Medicine 2000;161:527‐34. - PubMed
Simons 1997 {published data only}
-
- Malozowski S, Stadel BV, Pian LP. Comparison of beclomethasone, salmeterol, and placebo in children with asthma. New England Journal of Medicine 1998;339(10):704‐5. - PubMed
-
- SMS40065 (521/120 [SLPT10]). Efficacy and safety of long‐term inhaled salmeterol and beclomethasone dipropionate in corticosteroid‐naïve children with mild to moderate, chronic, stable asthma. http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
-
- Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate‐Salmeterol Xinafoate Study Group. New England Journal of Medicine 1997;337(23):1659‐65. - PubMed
-
- Simons FER, Dolovich J, Moote DW, Mazza JA, Lyttle B, Becker AB, et al. A one year placebo controlled study of the efficacy and safety of beclomethasone DP versus salmeterol in glucocorticoid naive children with asthma. Journal of Allergy and Clinical Immunology 1997;99:S402.
SLGA 3014 {unpublished data only}
-
- GlaxoSmithKline. Serevent submission to the FDA. http://www.fda.gov/cder/foi/nda/98/20692S1,2_Serevent_medr_P2.pdf 2001.
SLMF4002 {published data only}
-
- SLMF 4002. Efficacy and safety of salmeterol in patients with asthma controlled with inhaled corticosteroids. http://ctr.gsk.co.uk/Summary/salmeterol/IV_SLMF4002.pdf.
SMART 2006 {published data only}
-
- GlaxoSmithKline. Safety of Long‐Acting Beta‐Agonist Bronchodilators. http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005‐4148B1_01_01‐GSK‐Se... 2005.
-
- Knobil K, Yancey S, Kral K, Rickard K. Salmeterol Multicentre Asthma Research Trial (SMART): results from an interim analysis. Chest 2003;124:335s.
-
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, the SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129(1):15‐26. - PubMed
-
- Rickard KA. 2003 safety alert ‐ serevent (salmeterol xinafoate). www.fda.gov 2003.
-
- SLGA5011 SMART. SMART: a double‐blind, randomized, placebo‐controlled surveillance study of asthma event outcomes in subjects receiving either usual pharmacotherapy of asthma or usual pharmacotherapy plus salmeterol 42mcg twice daily. http://www.ctr.gsk.co.uk 2006.
SNS 1993 {published data only}
-
- SNS‐D920619. Serevent Nationwide Surveillance (SNS) Trial. http://ctr.gsk.co.uk/Summary/salmeterol/IV_SNS_D920619.pdf.
Von Berg 1998 {published data only}
-
- SLGB3019 (SLPT09). A multicentre, randomised, double‐blind, parallel group study comparing the efficacy and safety of inhaled salmeterol xinafoate 50 mcg bd with that of salbutamol 200 mcg to use “as required” from Diskhalers for twelve months in children with asthma. http://ctr.gsk.co.uk/Summary/salmeterol/III_SLGB3019.pdf.
-
- Berg A, Blic J, Rosa M. Regular salbutamol xinafoate compared with salbutamol as required in children with moderate asthma. American Journal of Respiratory and Critical Care Medicine 1996;4:A76.
-
- Berg A, Blic J, Rosa M, Kaad PH, Moorat A. A comparison of regular salmeterol vs 'as required' salbutamol therapy in asthmatic children. Respiratory Medicine 1998;92(2):292‐9. - PubMed
Weinstein 1998 {published data only}
-
- GlaxoSmithKline. Safety of Long‐Acting Beta‐Agonist Bronchodilators. www.fda.gov/ohrms/dockets/ac/05/briefing/2005‐4148B1_01_01‐GSK‐Serevent.pdf 2005.
-
- Pearlman DS, Bronsky E, Chervinsky P. Inhaled salmeterol powder compared with placebo administered over 12 weeks to children with mild to moderate asthma. American Journal of Respiratory and Critical Care Medicine 1996;153(4):A76.
-
- Weinstein S, Pearlman D, Bronsky E, Byrne A, Arledge T, Liddle R, et al. Efficacy of salmeterol xinafoate powder in children with chronic persistent asthma. Annals of Allergy, Asthma & Immunology 1998;81(1):51‐8. - PubMed
Wenzel 1998 {published data only}
-
- Cox F, Goodwin B, Wenzel S, Rickard K, Kalberg C, Emmett A. Asthma specific quality of life in patients treated with salmeterol or albuterol. Journal of Allergy and Clinical Immunology 1996;97:256.
-
- Goodwin R, Cox F, Lumry W, Rickard K, Kalberg C, Emmett A. The impact of salmeterol versus albuterol on disease specific quality of life in mild to moderate asthmatics. Journal of Allergy and Clinical Immunology 1996;97 (Pt 3):256.
-
- Wenzel SE, Lumry W, Manning M, Kalberg C, Cox F, Emmett A, et al. Efficacy, safety, and effects on quality of life of salmeterol versus albuterol in patients with mild to moderate persistent asthma. Annals of Allergy 1998;80(6):463‐70. - PubMed
Wolfe 2000 {published and unpublished data}
-
- SLGA3010. A randomized, double‐blind, double‐dummy, comparative clinical trial of salmeterol 50 mcg via the Diskus and salmeterol 50 mcg via the metered‐dose inhaler versus placebo for 12 weeks in adolescent and adult subjects with mild‐to‐moderate asthma. http://ctr.gsk.co.uk/Summary/salmeterol/III_SLGA3010.pdf.
-
- SLGA3011. A randomized, double‐blind, double‐dummy, comparative clinical trial of salmeterol 50 mcg via the Diskus and salmeterol 50 mcg via the metered‐dose inhaler versus placebo for twelve weeks in adolescent and adult subjects with mild‐to‐moderate asthma.. http://ctr.gsk.co.uk/Summary/salmeterol/III_SLGA3011.pdf.
-
- Wolfe J, Kreitzer S, Chervinsky P, Lawrence M, Wang Y, Reilly D, et al. Comparison of powder and aerosol formulations of salmeterol in the treatment of asthma. Annals of Allergy 2000;84(3):334‐40. - PubMed
References to studies excluded from this review
Bagnato 1996 {published data only}
-
- Bagnato GF, Mileto A, Gulli S, Oriti S, Cesare E, Cinquegrani M, et a, Acute cardiovascular effects of salmeterol in subjects with stable bronchial asthma. Monaldi Archives for Chest Disease 1996;51(4):275‐8. - PubMed
Beach 1992 {published data only}
-
- Beach JR, Young CL, Stenton SC, Avery AJ, Walters EH, Hendrick DJ. A comparison of the speeds of action of salmeterol and salbutamol in reversing methacholine‐induced bronchoconstriction. Pulmonary Pharmacology 1992;5(2):133‐5. - PubMed
Beach 1993 {published data only}
-
- Beach JR, Young CL, Harkawat R, Gardiner PV, Avery AJ, Coward GA, et al. Effect on airway responsiveness of six weeks treatment with salmeterol. Pulmonary Pharmacology 1993;6(2):155‐7. - PubMed
Blake 1999 {published data only}
-
- Blake K, Pearlman DS, Scott C, Wang Y, Stahl E, Arledge T. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of salmeterol powder with albuterol. Annals of Allergy, Asthma & Immunology 1999;82(2):205‐11. - PubMed
Bons 1992 {published data only}
-
- Bons J, Pappo M. Salmeterol and prolonged treatment of asthma: international clinical data. Revue des Maladies Respiratoires 1992;9 Suppl 1:R15‐8. - PubMed
Booth 1993 {published data only}
Boulet 1997b {published data only}
-
- Boulet LP, Turcotte H, Boutet M, Dube J, Gagnon M, Laviolette M. Influence of salmeterol on chronic and allergen‐induced airway inflammation in mild allergic asthma: a pilot study. Current Therapeutic Research, Clinical & Experimental 1997;58(4):240‐59.
Bousquet 1996 {published data only}
-
- Bousquet J, Aubert B, Bons J. Comparison of salmeterol with disodium cromoglycate in the treatment of adult asthma. Annals of Allergy, Asthma & Immunology 1996;76(2):189‐94. - PubMed
Brambilla 1994 {published data only}
-
- Brambilla C, Chastang C, Georges D, Bertin L. Salmeterol compared with slow‐release terbutaline in nocturnal asthma. A multicenter, randomized, double‐blind, double‐dummy, sequential clinical trial. French Multicenter Study Group. Allergy 1994;49(6):421‐6. - PubMed
Bronsky 1994 {published data only}
-
- Bronsky EA, Kemp JP, Orgel HA, Bierman CW, Tinkelman DG, As A, et al. A 1‐week dose‐ranging study of inhaled salmeterol in patients with asthma. Chest 1994;105(4):1032‐7. - PubMed
Bronsky 1999 {published data only}
-
- Bronsky EA, Pearlman DS, Pobiner BF, Scott C, Wang Y, Stahl E. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of two salmeterol powder delivery devices. Pediatrics 1999;104(3 Pt 1):501‐6. - PubMed
Busse 1999 {published data only}
-
- Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. Journal of Allergy and Clinical Immunology 1999;103(6):1075‐80. - PubMed
Cartier 1993 {published data only}
-
- Cartier A, Ghezzo H, L'Archeveque J, Trudeau C, Malo JL. Duration and magnitude of action of 50 and 100 micrograms of inhaled salmeterol in protecting against bronchoconstriction induced by hyperventilation of dry cold air in subjects with asthma. Journal of Allergy and Clinical Immunology 1993;92(3):488‐92. - PubMed
Castle 1992 {published data only}
-
- Castle WM. Review of salmeterol international safety data base. European Respiratory Journal 1992;5(Suppl 15):318S.
Cazzola 2002 {published data only}
-
- Cazzola M, Califano C, Perna F, D'Amato M, Terzano C, Matera MG, et al. Acute effects of higher than customary doses of salmeterol and salbutamol in patients with acute exacerbation of COPD. Respiratory Medicine 2002;96(10):790‐5. - PubMed
Ceugniet 1997 {published data only}
-
- Ceugniet F, Cauchefer F, Fragneaud C, Evano‐Celli I. Prophylactic treatment of exercise‐induced asthma in children: salmeterol or sodium cromoglycate single dose before exercise?. Annales De Pediatrie 1997;44(9):625‐34.
Cheung 1992 {published data only}
-
- Cheung D, Timmers MC, Zwinderman AH, Bel EH, Dijkman JH, Sterk PJ. Long‐term effects of a long‐acting beta 2‐adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. New England Journal of Medicine 1992;327(17):1198‐203. - PubMed
Chopra 2005 {published data only}
-
- Chopra N, Williams M, Rimmer M, Kahl L, Jenkins M, Teams SMOaSIS. Salmeterol HFA is as effective as salmeterol CFC in children and adults with persistent asthma. Respiratory Medicine 2005;99(Suppl 1):S1‐10. - PubMed
Cloosterman 2001 {published data only}
-
- Cloosterman SG, Bijl‐Hofland ID, Herwaarden CL, Akkermans RP, Elshout FJ, Folgering HT, et al. A placebo‐controlled clinical trial of regular monotherapy with short‐acting and long‐acting beta(2)‐agonists in allergic asthmatic patients. Chest 2001;119(5):1306‐15. - PubMed
Crompton 1999 {published data only}
-
- Crompton GK, Ayres JG, Basran G, Schiraldi G, Brusasco V, Eivindson A, et al. Comparison of oral bambuterol and inhaled salmeterol in patients with symptomatic asthma and using inhaled corticosteroids. American Journal of Respiratory and Critical Care Medicine 1999;159(3):824‐8. - PubMed
D'Alonzo 1995 {published data only}
-
- D'Alonzo GE. Efficacy of inhaled salmeterol in the treatment of asthma. European Respiratory Review 1995;5(27):128‐32.
D'Urzo 1998 {published data only}
-
- D'Urzo A, Chapman KR, Cartier A, Fitzgerald M, Hargreave FE, Tesarowski D. Safety and efficacy of salmeterol in a post‐marketing surveillance trial in asthmatics. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A402.
Dahl 1991 {published data only}
-
- Dahl R, Earnshaw JS, Palmer JB. Salmeterol: a four week study of a long‐acting beta‐adrenoceptor agonist for the treatment of reversible airways disease. European Respiratory Journal 1991;4(10):1178‐84. - PubMed
Dekhuijzen 2006 {published data only}
-
- Dekhuijzen PNR. Caution recommended in prescribing long‐acting beta2‐adrenergic agonists to patients with asthma. Nederlands Tijdschrift voor Geneeskunde 2006;150(16):889‐91. - PubMed
Demirkan 2000 {published data only}
-
- Demirkan K, Tolley E, Mastin T, Soberman J, Burbeck J, Self T. Salmeterol administration by metered‐dose inhaler alone vs metered‐dose inhaler plus valved holding chamber. Chest 2000;117(5):1314‐8. - PubMed
De Oliveira 1998 {published data only}
-
- Oliveira MA, Jardim JR, Faresin SM, Lucas SR, Nery LE. Efficacy of and tolerance to salmeterol compared to salbutamol in patients with bronchial asthma. Revista Da Associacao Medica Brasileira 1998;44(3):169‐75. - PubMed
Deykin 2007 {published data only}
Edelman 2000 {published data only}
-
- Edelman JM, Turpin JA, Bronsky EA, Grossman J, Kemp JP, Ghannam AF, et al. Oral montelukast compared with inhaled salmeterol to prevent exercise‐induced bronchoconstriction. A randomized, double‐blind trial. Annals of Internal Medicine 2000;132(2):97‐104. - PubMed
Eryonucu 2005 {published data only}
-
- Eryonucu B, Uzun K, Guler N, Tuncer M, Sezgi C. Comparison of the short‐term effects of salmeterol and formoterol on heart rate variability in adult asthmatic patients. Chest 2005;128(3):1136‐9. - PubMed
Faurschou 1994 {published data only}
-
- Faurschou P, Engel AM, Haanaes OC. Salmeterol in two different doses in the treatment of nocturnal bronchial asthma poorly controlled by other therapies. Allergy 1994;49(10):827‐32. - PubMed
Faurschou 1996 {published data only}
-
- Faurschou P, Steffensen I, Jacques L. Effect of addition of inhaled salmeterol to the treatment of moderate‐to‐severe asthmatics uncontrolled on high‐dose inhaled steroids. European Respiratory Study Group. European Respiratory Journal 1996;9(9):1885‐90. - PubMed
Fjellbirkeland 1994 {published data only}
-
- Fjellbirkeland L, Gulsvik A, Palmer JB. The efficacy and tolerability of inhaled salmeterol and individually dose‐titrated, sustained‐release theophylline in patients with reversible airways disease. Respiratory Medicine 1994;88(8):599‐607. - PubMed
Fuller 1995 {published data only}
-
- Fuller R. Safety of salmeterol in the treatment of asthma. European Respiratory Review 1995;5(27):133‐7.
GlaxoSmithKline 2005 {published data only}
-
- GlaxoSmithKline. A double‐blind evaluation of efficacy, safety, and tolerability of several single doses of salmeterol hydroxynaphthoate (GR33343G) compared with albuterol and placebo in asthmatic patients. GlaxoSmithKline Clinical Trial Register 2005.
Gongora 1991 {published data only}
-
- Gongora HC, Wisniewski AF, Tattersfield AE. A single‐dose comparison of inhaled albuterol and two formulations of salmeterol on airway reactivity in asthmatic subjects. American Review of Respiratory Disease 1991;144(3 Pt 1):626‐9. - PubMed
Gotz 1995 {published data only}
-
- Gotz MH. The efficacy and safety of inhaled salmeterol xinafoate (50 mcg bd) compared with salbutamol (200 mcg prn) in children with asthma. European Respiratory Journal 1995;8 Suppl 19:517S.
Gustafsson 1994 {published data only}
-
- Gustafsson PM, Von BA, Jenkins MM. Salmeterol 50 mcg twice daily in the treatment of mild‐to‐moderate asthma in childhood ‐ a comparison of two inhalation devices. European Journal of Clinical Research 1994;5:63‐73.
Harper 2001 {published data only}
-
- Harper TB, Stevens A, Clements D, Vandermeer A, Reisner C. Cardiac safety of salmeterol in 2 to 4 year old subjects with asthma. Journal of Allergy and Clinical Immunology 2001;107(2):S108.
Hermansson 1995 {published data only}
-
- Hermansson BA, Jenkins RJ. A 4‐week comparison of salmeterol and terbutaline in adult asthma. Allergy 1995;50(7):551‐8. - PubMed
Inoue 2007 {published data only}
-
- Inoue H, Komori M, Matsumoto T, Fukuyama S, Matsumura M, Nakano T, et al. Effects of salmeterol in patients with persistent asthma receiving inhaled corticosteroid plus theophylline. Respiration 2007; Vol. 74, issue 6:611‐6. - PubMed
Jartti 1998 {published data only}
-
- Jartti TT, Kaila TJ, Tahvanainen KU, Kuusela TA, Vanto TT, Valimaki IA. Altered cardiovascular autonomic regulation after salmeterol treatment in asthmatic children. Clinical Physiology 1998;18(4):345‐53. - PubMed
Jenkins 1991 {published data only}
-
- Jenkins MM, Hilton CJ, Kock JC, Palmer JB. Exacerbations of asthma in patients on salmeterol. Lancet 1991;337(8746):913‐4. - PubMed
Johnson 1994 {published data only}
-
- Johnson ME. A multicentre study to compare the efficacy and safety of salmeterol xinafoate and nedocromil sodium via metered‐dose inhalers in adults with mild‐to‐moderate asthma. European Journal of Clinical Research. 1994;5:75‐85.
Kemp 1993 {published data only}
-
- Kemp JP, Bierman CW, Cocchetto DM. Dose‐response study of inhaled salmeterol in asthmatic patients with 24‐hour spirometry and Holter monitoring. Annals of Allergy 1993;70(4):316‐22. - PubMed
Kirby 1995 {published data only}
Kraemer 1997 {published data only}
-
- Kraemer R, Dhank B. Long term efficacy and tolerability of salmeterol (50 µG BD) administered by pressurised inhaler propelled by propellants 11 and 12 or by an alternative propellant, GR106642X for a period of 12 months in children with reversible airways obstruction. European Respiratory Journal 1997;10(Suppl 25):222S.
Kurihara 1993 {published data only}
-
- Kurihara K, Mikawa H, Baba M, Ichikawa K, Masuda K, Yamada T, et al. Clinical study of salmeterol xinafoate (SN408) dry powder: comparative study between salmeterol xinafoate aerosol in children with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(10):2473‐87.
Langley 1998 {published data only}
-
- Langley SJ, Masterson CM, Batty EP, Woodcock A. Bronchodilator response to salbutamol after chronic dosing with salmeterol or placebo. European Respiratory Journal 1998;11(5):1081‐5. - PubMed
Langton Hewer 1995 {published data only}
-
- Langton Hewer S, Hobbs J, French D, Lenney W. Pilgrims progress: the effect of salmeterol in older children with chronic severe asthma. Respiratory Medicine 1995;89:435‐40. - PubMed
Lemaigre 2006 {published data only}
-
- Lemaigre V, Bergh O, Smets A, Peuter S, Verleden GM. Effects of long‐acting bronchodilators and placebo on histamine‐induced asthma symptoms and mild bronchus obstruction. Respiratory Medicine 2006;100(2):348‐53. - PubMed
Lemanske 2001 {published data only}
-
- Lemanske RF Jr, Sorkness CA, Mauger EA, Lazarus SC, Boushey HA, Fahy JV, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA 2001;285(20):2594‐603. - PubMed
Lotvall 1998 {published data only}
-
- Lotvall J, Lunde H, Svedmyr N. Onset of bronchodilation and finger tremor induced by salmeterol and salbutamol in asthmatic patients. Japanese Journal of Urology 1998;89(1):43‐9. - PubMed
Lurie 2005 {published data only}
-
- Lurie P, Wolfe SM. Misleading data analyses in salmeterol (SMART) study. Lancet 2005;366(9493):1261‐2. - PubMed
Martin 1999 {published data only}
-
- Martin RJ, Kraft M, Beaucher WN, Kiechel F, Sublett JL, LaVallee N, et al. Comparative study of extended release albuterol sulfate and long‐acting inhaled salmeterol xinafoate in the treatment of nocturnal asthma. Annals of Allergy, Asthma, & Immunology 1999;83(2):121‐6. - PubMed
Meier 1997 {published data only}
Mikawa 1993 {published data only}
-
- Mikawa H, Mayumi M, Kimata H, Baba M, Ichikawa K, Matsumoto S, et al. Clinical study of salmeterol xinafoate (SN408) aerosol: comparative study by multiple administration in children with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(Suppl 4):179‐99.
Miyamoto 1993a {published data only}
-
- Miyamoto T, Takishima T, Makino S, Kabe J, Takahashi T, Yamakido M, et al. Clinical study of salmeterol xinafoate (SN408) aerosol: double‐blind parallel study between procaterol tablet in patients with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(11):2671‐99.
Miyamoto 1993b {published data only}
-
- Miyamoto T, Takishima T, Makino S, Kabe J, Takahashi T, Yamakido M, et al. Clinical study of salmeterol xinafoate (SN408) aerosol: examination on optimal dose by multiple administration in patients with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(Suppl 4):81‐111.
Miyamoto 1993c {published data only}
-
- Miyamoto T, Takahashi T, Kabe J, Makino S. Clinical study of salmeterol xinafoate (SN408) aerosol: examination on bronchodilation by single administration in patients with bronchial asthma: examination on bronchodilation by single administration in patients with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(Suppl 4):23‐48.
Miyamoto 1993d {published data only}
-
- Miyamoto T, Takahashi T, Kabe J, Makino S. Clinical study of salmeterol xinafoate (SN408) dry powder: examination on bronchodilation by single administration in patients with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(Suppl 4):219‐41.
Miyamoto 1993e {published data only}
-
- Miyamoto T, Makino S, Kabe J, Takahashi T, Nakashima M. Clinical study of salmeterol xinafoate (SN408) aerosol: comparative study by multiple administration in patients with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9:49‐79.
Morgan 1994 {published data only}
-
- Morgan M. A comparison of the efficiency of salmeterol xinofoate with oral salbutamol controlled release against exercise induced asthma in adult asthmatics. National Research Register (https://portal.nihr.ac.uk/Pages/NRRArchiveSearch.aspx) 1994. [N0123137694]
Muir 1992 {published data only}
-
- Muir JF, Georges D. Usefulness of salmeterol in nocturnal asthma: a comparative study with theophylline‐ketotifen association. A French multicenter group. Revue des Maladies Respiratoires 1992;9(Suppl 1):R23‐6. - PubMed
Nathan 1995 {published data only}
-
- Nathan RA, Seltzer JM, Kemp JP, Chervinsky P, Alexander WJ, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma & Immunology 1995;75(3):243‐8. - PubMed
Nelson 1999 {published data only}
-
- Nelson HS, Berkowitz RB, Tinkelman DA, Emmett AH, Rickard KA, Yancey SW. Lack of subsensitivity to albuterol after treatment with salmeterol in patients with asthma. American Journal of Respiratory and Critical Care Medicine 1999;159(5 Pt 1):1556‐61. - PubMed
Nelson 2001 {published data only}
-
- Nelson HS, Nathan RA, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in asthmatic patients using concomitant inhaled corticosteroids. Medscape General Medicine 2001;3(4):3. - PubMed
Nishima 1993 {published data only}
-
- Nishima S, Odajima H, Origasa H. Clinical study of salmeterol xinafoate (SN408) dry powder: bioequivalency study between salmeterol xinafoate aerosol in children with bronchial asthma by single dose crossover method. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(9):2133‐46.
Nishiyama 2006 {published data only}
-
- Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Kato K, Kume H, et al. Comparison of the effects of tulobuterol patch and salmeterol in moderate to severe asthma. Clinical and Experimental Pharmacology and Physiology 2006;33(11):1016‐21. - PubMed
Norhaya 1999 {published data only}
-
- Norhaya MR, Yap TM, Zainudin BMZ. Addition of inhaled salmeterol to inhaled corticosteroids in patients with poorly controlled nocturnal asthma. Respirology 1999;4(1):77‐81. - PubMed
Nutini 1998 {published data only}
-
- Nutini S, Martini T, Righi R. Long‐term treatment of asthmatic patients with salmeterol vs slow‐release theophylline. Respiratory Medicine 1998;92(4):683‐90. - PubMed
Orgel 1985 {published data only}
-
- Orgel HA, Kemp JP, Tinkelman DG, Webb DR, Jr. Bitolterol and albuterol metered‐dose aerosols: comparison of two long‐acting beta 2‐adrenergic bronchodilators for treatment of asthma. Journal of Allergy and Clinical Immunology 1985;75(1 Pt 1):55‐62. - PubMed
Ortiz 2002 {published data only}
-
- Ortiz G, Menendez R. The effects of inhaled albuterol and salmeterol in 2‐ to 5‐year‐old asthmatic children as measured by impulse oscillometry. Journal of Asthma 2002;39(6):531‐6. - PubMed
Paggiaro 1996 {published data only}
-
- Paggiaro PL, Giannini D, Di FA, Testi R. Comparison of inhaled salmeterol and individually dose‐titrated slow‐ release theophylline in patients with reversible airway obstruction. European Respiratory Journal 1996;9(8):1689‐95. - PubMed
Palmer 1992 {published data only}
-
- Palmer JB, Stuart AM, Shepherd GL, Viskum K. Inhaled salmeterol in the treatment of patients with moderate to severe reversible obstructive airways disease ‐ a 3‐month comparison of the efficacy and safety of twice‐daily salmeterol 100 micrograms with salmeterol 50 micrograms. Respiratory Medicine 1992;86(5):409‐17. - PubMed
Pascoe 2006 {published data only}
-
- Pascoe S, Knowles J, Glasbrenner M, Duvauchelle T, Fuhr R, Brookman J. Efficacy and safety of therapeutic and supratherapeutic doses of indacaterol compared to salmeterol and salbutamol in mild to moderate asthma [Abstract]. European Respiratory Journal 2006; Vol. 28, issue Suppl 50:207s [P1238].
Pastorello 1998 {published data only}
-
- Pastorello EA, Mauro M, Incorvaia C. Comparison of efficacy and safety of inhaled salmeterol and slow‐release oral theophylline in patients with moderate/severe asthma. Internista 1998;6(2):101‐7.
Pearlman 1999 {published data only}
-
- Pearlman DS, Stricker W, Weinstein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: a study comparing monotherapy and combination therapy in asthma. Annals of Allergy, Asthma, & Immunology 1999;82(3):257‐65. - PubMed
Peslis 1994 {published data only}
-
- Peslis N, Weber HH, Kettner J. Salmeterol and fenoterol ‐ comparison of efficacy and safety in patients with reversible obstructive airways disease. European Respiratory Journal 1994;7(Suppl 18):112s.
Peters 2000 {published data only}
-
- Peters JI, Shelledy DC, Jones AP Jr, Lawson RW, Davis CP, LeGrand TS. A randomized, placebo‐controlled study to evaluate the role of salmeterol in the in‐hospital management of asthma. Chest 2000;118(2):313‐20. - PubMed
Pohunek 2004 {published data only}
-
- Pohunek P, Matulka M, Rybnicek O, Kopriva F, Honomichlova H, Svobodova T. Dose‐related efficacy and safety of formoterol (Oxis) Turbuhaler compared with salmeterol Diskhaler in children with asthma. Pediatric Allergy and Immunology 2004;15(1):32‐9. - PubMed
Pollard 1997 {published data only}
-
- Pollard SJ, Spector SL, Yancey SW, Cox FM, Emmett A. Salmeterol versus theophylline in the treatment of asthma. Annals of Allergy, Asthma, & Immunology 1997;78(5):457‐64. - PubMed
Prieto 2002 {published data only}
-
- Prieto L, Gutierrez V, Torres V, Uixera S, Marin J. Effect of salmeterol on seasonal changes in airway responsiveness and exhaled nitric oxide in pollen‐sensitive asthmatic subjects. Chest 2002;122(3):798‐805. - PubMed
Revill 1998 {published data only}
-
- Revill SM, Morgan MD. The cardiorespiratory response to submaximal exercise in subjects with asthma following pretreatment with controlled release oral salbutamol and high‐dose inhaled salmeterol. Respiratory Medicine 1998;92(8):1053‐8. - PubMed
Rhee 1997 {published data only}
-
- Rhee YK. A comparison of salmeterol with salbutamol inhalation in treatment of mild to moderate asthma. Tuberculosis and Respiratory Diseases 1997;44(4):815‐21.
Ringbaek 1996 {published data only}
-
- Ringbaek TJ, Soes‐Petersen U, Christensen M, Iversen ET, Rasmussen FV. Salmeterol improves the control of disease in patients with moderate asthma. A comparative study of inhaled salmeterol 50 mg and salbutamol depot tablets 8 mg, both administered twice daily. Ugeskrift for Laeger 1996;158(27):3940‐3. - PubMed
Ringdal 1995 {published data only}
-
- Ringdal N, Bateman E, Laitinen L, Sykes AP. Efficacy and safety of salmeterol 50 mcg via a pressurised inhaler formulated with GR106642X. American Journal of Respiratory and Critical Care Medicine 1995;151(4 Pt 2):A57.
Ritz 1997 {published data only}
-
- Ritz M, Thorens JB, Arnold‐Ketterer M, Chevrolet JC. Effects of inhaled salmeterol and salbutamol (albuterol) on morning dips compared in intensive care patients recovering from an acute severe asthma attack. Intensive Care Medicine 1997;23(12):1225‐30. - PubMed
Roberts 1999 {published data only}
-
- Roberts JA, Bradding P, Britten KM, Walls AF, Wilson S, Gratziou C, et al. The long‐acting beta2‐agonist salmeterol xinafoate: effects on airway inflammation in asthma. European Respiratory Journal 1999;14(2):275‐82. - PubMed
Sano 1993 {published data only}
-
- Sano Y, Miyamoto Y, Arai Y, Tanaka Y, Akiyama N, Origasa H. Clinical study of salmeterol xinafoate (SN408) dry powder: bioequivalency study between salmeterol xinafoate aerosol in patients with bronchial asthma by single dose crossover method. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(9):2105‐18.
Schaanning 1996 {published data only}
-
- Schaanning J, Vilsvik J, Henriksen AH, Bratten G. Efficacy and duration of salmeterol powder inhalation in protecting against exercise‐induced bronchoconstriction. Annals of Allergy, Asthma, & Immunology 1996;76(1):57‐60. - PubMed
Shaheen 1994 {published data only}
-
- Shaheen MZ, Ayres JG, Benincasa C. Incidence of acute decreases in peak expiratory flow following the use of metered‐dose inhalers in asthmatic patients. European Respiratory Journal 1994;7(12):2160‐4. - PubMed
Shepherd 1991 {published data only}
-
- Shepherd GL, Jenkins WJ, Alexander J. Asthma exacerbations in patients taking regular salmeterol, or salbutamol for symptoms. Lancet 1991;337(8754):1424. - PubMed
SLGL24 {published data only}
-
- SLGL24. A double‐blind, randomised, parallel group study to investigate the efficacy of inhaled salmeterol 50 mcg administered twice daily compared with salbutamol 200 mcg administered four times daily in patients with poorly controlled nocturnal asthma. http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
Stahl 1999 {published data only}
-
- Stahl E, Svensson K, Crompton GK. Bambuterol is a more cost‐effective treatment strategy than salmeterol in asthmatic patients with nocturnal symptoms. Journal of Medical Economics 1999;2:117‐22.
Starke 1996 {published data only}
-
- Starke ID, Luce P. The efficacy and safety of inhaled salmeterol 50 microg bd in older patients with reversible airflow obstruction. Age and Ageing 1996;25(1):67‐71. - PubMed
Storms 2004 {published data only}
-
- Storms W, Chervinsky P, Ghannam AF, Bird S, Hustad CM, Edelman JM, et al. A comparison of the effects of oral montelukast and inhaled salmeterol on response to rescue bronchodilation after challenge. Respiratory Medicine 2004;98(11):1051‐62. - PubMed
Szczeklik 1998 {published data only}
-
- Szczeklik A, Dworski R, Mastalerz L, Prokop A, Sheller JR, Nizankowska E, et al. Salmeterol prevents aspirin‐induced attacks of asthma and interferes with eicosanoid metabolism. American Journal of Respiratory and Critical Care Medicine 1998;158(4):1168‐72. - PubMed
Taguchi 1993 {published data only}
-
- Taguchi O, Ibata H, Suzuki S, Miyamoto T. Clinical study of salmeterol xinafoate (SN408) in a regular inhalation therapy in patients with bronchial asthma: comparative evaluation with azelastine regarding its effect on bronchial hyperresponsiveness. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(11):2711‐23.
Taylor 1992 {published data only}
-
- Taylor IK, O'Shaughnessy KM, Choudry NB, Adachi M, Palmer JB, Fuller RW. A comparative study in atopic subjects with asthma of the effects of salmeterol and salbutamol on allergen‐induced bronchoconstriction, increase in airway reactivity, and increase in urinary leukotriene E4 excretion. Journal of Allergy & Clinical Immunology 1992;89(2):575‐83. - PubMed
Taylor 1998 {published data only}
Taylor 2000a {published data only}
Taylor 2000b {published data only}
-
- Taylor DR, Hancox RJ, McRae W, Cowan JO, Flannery EM, McLachlan CR, et al. The influence of polymorphism at position 16 of the beta2‐adrenoceptor on the development of tolerance to beta‐agonist. Journal of Asthma 2000;37(8):691‐700. - PubMed
Thompson 1994 {published data only}
-
- Thompson P, Bellesis M. Long‐term safety and efficacy of salmeterol in nocturnal asthmatics. Australian & New Zealand Journal of Medicine 1994;24:459.
Tomac 1996 {published data only}
-
- Tomac N, Tuncer A, Saraclar Y, Adalioglu G. Efficacy of salmeterol in the treatment of childhood asthma. Acta Paediatrica Japonica 1996;38(5):489‐94. - PubMed
Ukena 1997 {published data only}
-
- Ukena D, Koper I, Braun H, Leutz M, Schlimmer P, Sybrecht GW. Therapy of nocturnal asthma: salmeterol versus nocturnal administration of retard theophylline‐‐comparison of effectiveness and tolerance. Pneumologie 1997;51(3):317‐23. - PubMed
Ullman 1990 {published data only}
-
- Ullman A, Hedner J, Svedmyr N. Inhaled salmeterol and salbutamol in asthmatic patients. An evaluation of asthma symptoms and the possible development of tachyphylaxis. American Review of Respiratory Disease 1990;142(3):571‐5. - PubMed
Venables 1992 {published data only}
-
- Venables T. A multicentre study in general practice to compare the efficacy and tolerability of inhaled salmeterol xinafoate and terbutaline in the treatment of asthma. British Journal of Clinical Research 1992;3:125‐36.
Verberne 1993 {published data only}
-
- Verberne AA, Hop WC, Bos AB, Kerrebijn KF. Effect of a single dose of inhaled salmeterol on baseline airway caliber and methacholine‐induced airway obstruction in asthmatic children. Journal of Allergy and Clinical Immunology 1993;91(1 Pt 1):127‐34. - PubMed
Verberne 1998 {published data only}
-
- SLGB4014 (SLPT15). Placebo controlled study during one year comparing the addition of salmeterol with an increase of the dose of the inhaled corticosteroid in asthmatic children already on treatment with inhaled corticosteroids. http://ctr.gsk.co.uk/Summary/salmeterol/IV_SLGB4014.pdf.
-
- Verberne AA, Frost C, Roorda RJ, Laag H, Kerrebijn KF. One year treatment with salmeterol compared with beclomethasone in children with asthma. The Dutch Paediatric Asthma Study Group. American Journal of Respiratory & Critical Care Medicine 1997;156(3 Pt 1):688‐95. - PubMed
Verini 1998 {published data only}
-
- Verini M, Verrotti A, Greco R, Chiarelli F. Comparison of the bronchodilator effect of inhaled short‐ and long‐acting beta2‐agonists in children with bronchial asthma. A randomised trial. Clinical Drug Investigation 1998;16(1):19‐24. - PubMed
Villaran 1999 {published data only}
-
- Villaran C, O'Neill SJ, Helbling A, Noord JA, Lee TH, Chuchalin AG, et al. Montelukast versus salmeterol in patients with asthma and exercise‐induced bronchoconstriction. Montelukast/Salmeterol Exercise Study Group. Journal of Allergy & Clinical Immunology 1999;104(3 Pt 1):547‐53. - PubMed
Weersink 1997 {published data only}
-
- Weersink EJ, Zomeren EH, Koeter GH, Postma DS. Treatment of nocturnal airway obstruction improves daytime cognitive performance in asthmatics. American Journal of Respiratory & Critical Care Medicine 1997;156(4 Pt 1):1144‐50. - PubMed
Weiner 2003 {published data only}
-
- Weiner P, Magadle R, Beckerman M, Berar‐Yanay N. The effect of time on the perception of dyspnea following inhaled long‐acting bronchodilator. Harefuah 2003;142(5):342‐4, 398. - PubMed
Weinstein 1997 {published data only}
-
- Weinstein S, Chervinsky P, Pollard SJ, Bronsky EA, Nathan RA, Prenner B, et al. A one‐week dose‐ranging study of inhaled salmeterol in children with asthma. Journal of Asthma 1997;34(1):43‐52. - PubMed
Wiegand 1999 {published data only}
-
- Wiegand L, Mende CN, Zaidel G, Zwillich CW, Petrocella VJ, Yancey SW, et al. Salmeterol vs theophylline: sleep and efficacy outcomes in patients with nocturnal asthma. Chest 1999;115(6):1525‐32. - PubMed
Wilding 1997 {published data only}
Williams 1998 {published data only}
Zarkovic 1998 {published data only}
-
- Zarkovic J, Gotz MH, Holgate ST, Taak NK. Effect of long‐term regular salmeterol treatment in children with moderate asthma. Clinical Drug Investigation 1998;15(3):169‐175.
Zimmermann 2003 {published data only}
-
- Zimmermann T, Gulyas A, Bauer CP, Steinkamp G, Trautmann M. Salmeterol versus sodium cromoglycate for the protection of exercise induced asthma in children‐‐a randomised cross‐over study. European Journal of Medical Research 2003;8(9):428‐34. - PubMed
Additional references
Altman 2003
Anderson 2006
-
- Anderson GP. Current issues with beta(2)‐adrenoceptor agonists ‐ pharmacology and molecular and cellular mechanisms. Clinical Reviews in Allergy & Immunology 2006; Vol. 31, issue 2‐3:119‐30. - PubMed
Arnold 1985
-
- Arnold JMO, Oconnor PC, Riddell JG, Harron DWG, Shanks RG, McDevitt DG. Effects of the beta‐2‐adrenoceptor antagonist ici‐118,551 on exercise tachycardia and isoprenaline‐induced beta‐adrenoceptor responses in man. British Journal of Clinical Pharmacology 1985; Vol. 19, issue 5:619‐30. - PMC - PubMed
Barnes 1993
-
- Barnes PJ. Beta‐adrenoceptors on smooth‐muscle, nerves and inflammatory cells. Life Sciences 1993; Vol. 52, issue 26:2101‐9. - PubMed
Barnes 1995
-
- Barnes PJ. Beta‐adrenergic receptors and their regulation. American Journal of Respiratory and Critical Care Medicine 1995; Vol. 152, issue 3:838‐60. - PubMed
Bennett 1994
Benson 1948
-
- Benson RL, Perlman F. Clinical effects of epinephrine by inhalation ‐ a survey. Journal of Allergy 1948; Vol. 19, issue 2:129‐40. - PubMed
Bergman 1969
-
- Bergman J, Persson H, Wetterli K. Two new groups of selective stimulants of adrenergic beta‐receptors. Experientia 1969; Vol. 25, issue 9:899‐901. - PubMed
Bijl‐Hofland 2001
-
- Bijl‐Hofland ID, Cloosterman SG, Folgering HT, Elshout FJ, Weel C, Schayck CP. Inhaled corticosteroids, combined with long‐acting beta(2)‐agonists, improve the perception of bronchoconstriction in asthma. American Journal of Respiratory and Critical Care Medicine 2001; Vol. 164, issue 5:764‐9. - PubMed
Boushey 1980
-
- Boushey HA, Holtzman MJ, Sheller JR, Nadel JA. Bronchial hyperreactivity. American Review of Respiratory Disease 1980; Vol. 121, issue 2:389‐413. [0003‐0805: (Print)] - PubMed
Bousquet 1990
-
- Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, et al. Eosinophilic inflammation in asthma. New England Journal of Medicine 1990; Vol. 323, issue 15:1033‐9. - PubMed
Bousquet 2000
-
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. American Journal Respiratory Critical Care Medicine 2000; Vol. 161, issue 5:1720‐45. - PubMed
Brewster 1990
-
- Brewster CEP, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial‐asthma. American Journal of Respiratory Cell and Molecular Biology 1990; Vol. 3, issue 5:507‐11. - PubMed
Brown 1983
-
- Brown MJ, Brown DC, Murphy MB. Hypokalemia from beta‐2‐receptor stimulation by circulating epinephrine. New England Journal of Medicine 1983; Vol. 309, issue 23:1414‐9. - PubMed
Brusasco 1998
Burgess 1991
-
- Burgess CD, Windom HH, Pearce N, Marshall S, Beasley R, Siebers RWL, et al. Lack of evidence for beta‐2 receptor selectivity ‐ a study of metaproterenol, fenoterol, isoproterenol, and epinephrine in patients with asthma. American Review of Respiratory Disease 1991; Vol. 143, issue 2:444‐6. - PubMed
Burggraaf 2001
Carroll 1993
-
- Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. American Review of Respiratory Disease 1993; Vol. 147, issue 2:405‐10. - PubMed
Cates 2009
Cockcroft 1993
-
- Cockcroft DW, McParland CP, Britto SA, Swystun VA, Rutherford BC. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet 1993; Vol. 342, issue 8875:833‐7. - PubMed
Cockcroft 2006
-
- Cockcroft D. Airway hyperresponsiveness as a determinant of the early asthmatic response to inhaled allergen. Journal of Asthma 2006; Vol. 43, issue 3:175‐8. - PubMed
Collins 1969
Crane 1989
-
- Crane J, Pearce N, Flatt A, Burgess C, Jackson R, Kwong T, et al. Prescribed fenoterol and death from asthma in New Zealand, 1981‐83 ‐ case‐control study. Lancet 1989; Vol. 1, issue 8644:917‐22. - PubMed
Cullum 1969
Delea 2008
-
- Delea TE, Hagiwara M, Stanford RH, Stempel DA. Effects of fluticasone propionate/salmeterol combination on asthma‐related health care resource utilization and costs and adherence in children and adults with asthma. Clinical Therapeutics 2008;30(3):560‐71. - PubMed
Ducharme 2006
Dunnill 1960
Ebina 1993
-
- Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial‐asthma ‐ a 3‐d morphometric studyY. American Review of Respiratory Disease 1993; Vol. 148, issue 3:720‐6. - PubMed
Gay 1949
-
- Gay LN, Long JW. Clinical evaluation of isopropylepinephrine in management of bronchial asthma. Journal of the American Medical Association 1949; Vol. 139, issue 7:452‐7. - PubMed
Grainger 1991
Greenstone 2005
-
- Greenstone IR, Ni Chroinin M, Masse V, Danish A, Magdalinos H, Zhang X, et al. Combination of inhaled long‐acting beta2‐agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005533] - DOI - PubMed
Guhan 2000
Haley 1998
-
- Haley KJ, Drazen JM. Inflammation and airway function in asthma ‐ what you see is not necessarily what you get. American Journal of Respiratory and Critical Care Medicine 1998; Vol. 157, issue 1:1‐3. - PubMed
Hall 1989
-
- Hall JA, Petch MC, Brown MJ. Intracoronary injections of salbutamol demonstrate the presence of functional beta‐2‐adrenoceptors in the human‐heart. Circulation Research 1989; Vol. 65, issue 3:546‐53. - PubMed
Hanania 2002
-
- Hanania NA, Sharfkhaneh A, Barber R, Dickey BF. beta‐agonist intrinsic efficacy ‐ measurement and clinical significance. American Journal of Respiratory and Critical Care Medicine 2002; Vol. 165, issue 10:1353‐8. - PubMed
Hanania 2007
-
- Hanania NA, Moore RH, Zimmerman JL, Miller CT, Bag R, Sharafkhaneh A, et al. The role of intrinsic efficacy in determining response to beta(2)‐agonist in acute severe asthma. Respiratory Medicine 2007; Vol. 101, issue 5:1007‐14. - PubMed
Hancox 1999
-
- Hancox RJ, Aldridge RE, Cowan JO, Flannery EM, Herbison GP, McLachlan CR, et al. Tolerance to beta‐agonists during acute bronchoconstriction. European Respiratory Journal 1999; Vol. 14, issue 2:283‐7. - PubMed
Hancox 2006a
-
- Hancox RJ. Concluding remarks: can we explain the association of beta‐agonists with asthma mortality? A hypothesis. Clinical Reviews in Allergy and Immunology 2006; Vol. 31, issue 2‐3:279‐88. - PubMed
Hancox 2006b
-
- Hancox RJ. Interactions between corticosteroids and beta2‐agonists. Clinical Reviews in Allergy and Immunology 2006; Vol. 31, issue 2‐3:231‐46. - PubMed
Haney 2006
-
- Haney S, Hancox RJ. Recovery from bronchoconstriction and bronchodilator tolerance. Clinical Reviews in Allergy & Immunology 2006; Vol. 31, issue 2‐3:181‐96. - PubMed
Harvey 1982
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Jones 2001
-
- Jones SL, Cowan JO, Flannery EM, Hancox RJ, Herbison GP, Taylor DR. Reversing acute bronchoconstriction in asthma: the effect of bronchodilator tolerance after treatment with formoterol. European Respiratory Journal 2001; Vol. 17, issue 3:368‐73. - PubMed
Konzett 1940
-
- Konzett H. Neue broncholytisch hochwirksame Körper der Adrenalinreihe. Naunyn‐Schmiedeberg's Archives of Pharmacology 1940; Vol. 197:27‐40.
Kuyper 2003
-
- Kuyper LM, Pare PD, Hogg JC, Lambert RK, Ionescu D, Woods R, et al. Characterization of airway plugging in fatal asthma. American Journal of Medicine 2003; Vol. 115, issue 1:6‐11. - PubMed
Lee 2003
Lipworth 1989
-
- Lipworth BJ, Struthers AD, McDevitt DG. Tachyphylaxis to systemic but not to airway responses during prolonged therapy with high‐dose inhaled salbutamol in asthmatics. American Review of Respiratory Disease 1989; Vol. 140, issue 3:586‐92. - PubMed
Lipworth 1992
Lipworth 1997
-
- Lipworth BJ. Airway subsensitivity with long‐acting beta 2‐agonists: is there cause for concern?. Drug Safety 1997;16(5):295‐308. - PubMed
Lipworth 2000
-
- Lipworth BJ, Aziz I. Bronchodilator response to albuterol after regular formoterol and effects of acute corticosteroid administration. Chest 2000; Vol. 117, issue 1:156‐62. - PubMed
Martinez 2006
-
- Martinez FD. Serious adverse events and death associated with treatment using long‐acting beta‐agonists. Clinical Reviews in Allergy & Immunology 2006; Vol. 31, issue 2‐3:269‐78. - PubMed
McDevitt 1974
McIvor 1998
-
- McIvor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. American Journal of Respiratory and Critical Care Medicine 1998; Vol. 158, issue 3:924‐30. - PubMed
Miranda 2004
-
- Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. Journal of Allergy and Clinical Immunology 2004; Vol. 113, issue 1:101‐8. - PubMed
Morrison 1993
-
- Morrison KJ, Gao Y, Vanhoutte PM. Beta‐adrenoceptors and the epithelial layer in airways. Life Sciences 1993; Vol. 52, issue 26:2123‐30. - PubMed
Nelson 1977
-
- Nelson HS, Raine D, Doner HC, Posey WC. Subsensitivity to bronchodilator action of albuterol produced by chronic administration. American Review of Respiratory Disease 1977; Vol. 116, issue 5:871‐8. - PubMed
Ni Chroinin 2004
Ni Chroinin 2005
-
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, et al. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005535] - DOI - PubMed
Ordonez 2001
-
- Ordonez CL, Khashayar R, Wong HH, Ferrando RON, Wu R, Hyde DM, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. American Journal of Respiratory and Critical Care Medicine 2001; Vol. 163, issue 2:517‐23. - PubMed
Palmqvist 1999
-
- Palmqvist M, Ibsen T, Mellen A, Lotvall J. Comparison of the relative efficacy of formoterol and salmeterol in asthmatic patients. American Journal of Respiratory and Critical Care Medicine 1999; Vol. 160, issue 1:244‐9. - PubMed
Pearce 1990
Pearce 2001
-
- Pearce N BR, Crane J, Burgess C. Epidemiology of asthma mortality. In Asthma and Rhinitis. Oxford: Blackwell Scientific 2001 2001:56‐69.
Pearce 2007
-
- Pearce N. Adverse Reactions: The Fenoterol Story. 1st Edition. Auckland: Auckland University Press, 2007:215.
Phillips 1990
-
- Phillips GD, Finnerty JP, Holgate ST. Comparative protective effect of the inhaled beta‐2‐agonist salbutamol (albuterol) on bronchoconstriction provoked by histamine, methacholine, and adenosine 5'‐monophosphate in asthma. Journal of Allergy and Clinical Immunology 1990; Vol. 85, issue 4:755‐62. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ringdal 1998
-
- Ringdal N, Derom E, Wahlin‐Boll E, Pauwels R. Onset and duration of action of single doses of formoterol inhaled via Turbuhaler (R). Respiratory Medicine 1998; Vol. 92, issue 8:1017‐21. - PubMed
Salpeter 2006
-
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting beta‐agonists on severe asthma exacerbations and asthma‐related deaths. Annals of Internal Medicine 2006;144(12):904‐12. - PubMed
Sears 1990
-
- Sears MR, Taylor DR, Print CG, Lake DC, Li QQ, Flannery EM, et al. Regular inhaled beta‐agonist treatment in bronchial‐asthma. Lancet 1990; Vol. 336, issue 8728:1391‐6. - PubMed
Simonsson 1967
Sovani 2008
Speizer 1968
Stolley 1972
-
- Stolley PD. Asthma mortality. Why the United States was spared an epidemic of deaths due to asthma. American Review of Respiratory Disease 1972; Vol. 105, issue 6:883‐90. - PubMed
Tattersfield 2006
-
- Tattersfield AE. Current issues with beta2‐adrenoceptor agonists: historical background. Clinical Reviews in Allergy & Immunology 2006; Vol. 31, issue 2‐3:107‐18. - PubMed
Tonnel 2001
-
- Tonnel AB, Gosset P, Tillie‐Leblond I. Characteristics of the inflammatory response in bronchial lavage fluids from patients with status asthmaticus. International Archives of Allergy and Immunology 2001; Vol. 124, issue 1‐3:267‐71. - PubMed
Van der Woude 2001
Van Noord 1996
-
- Noord JA, Smeets JJ, Raaijmakers JAM, Bommer AM, Maesen FPV. Salmeterol versus formoterol in patients with moderately severe asthma: onset and duration of action. European Respiratory Journal 1996;9(8):1684‐8. - PubMed
Vignola 1993
-
- Vignola AM, Campbell AM, Chanez P, Bousquet J, Paullacoste P, Michel FB, et al. HLA‐DR and ICAM‐1 expression on bronchial epithelial‐cells in asthma and chronic bronchitis. American Review of Respiratory Disease 1993; Vol. 148, issue 3:689‐94. - PubMed
Walters 2002
Walters 2007
Weber 1982
-
- Weber RW, Smith JA, Nelson HS. Aerosolized terbutaline in asthmatics ‐ development of subsensitivity with long‐term administration. Journal of Allergy and Clinical Immunology 1982; Vol. 70, issue 6:417‐22. - PubMed
Wiggs 1990
-
- Wiggs BR, Moreno R, Hogg JC, Hilliam C, Pare PD. A model of the mechanics of airway narrowing. Journal of Applied Physiology 1990; Vol. 69, issue 3:849‐60. - PubMed
Wilson 1981
-
- Wilson JD, Sutherland DC, Thomas AC. Has the change to beta‐agonists combined with oral theophylline increased cases of fatal asthma. Lancet 1981; Vol. 1, issue 8232:1235‐7. - PubMed
Wong 1990
-
- Wong CS, Pavord ID, Williams J, Britton JR, Tattersfield AE. Bronchodilator, cardiovascular, and hypokalemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet 1990; Vol. 336, issue 8728:1396‐9. - PubMed
Woodruff 2004
-
- Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. American Journal of Respiratory and Critical Care Medicine 2004; Vol. 169, issue 9:1001‐6. - PubMed
Yates 1996
-
- Yates DH, Kharitonov SA, Barnes PJ. An inhaled glucocorticoid does not prevent tolerance to the bronchoprotective effect of a long‐acting inhaled beta(2)‐agonist. American Journal of Respiratory and Critical Care Medicine 1996; Vol. 154, issue 6:1603‐7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

