Electronic structure and reactivity of three-coordinate iron complexes
- PMID: 18646779
- PMCID: PMC2587011
- DOI: 10.1021/ar700267b
Electronic structure and reactivity of three-coordinate iron complexes
Abstract
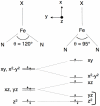
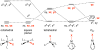
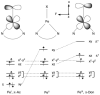
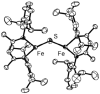
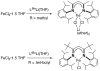
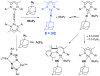
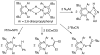
[Reaction: see text]. The identity and oxidation state of the metal in a coordination compound are typically thought to be the most important determinants of its reactivity. However, the coordination number (the number of bonds to the metal) can be equally influential. This Account describes iron complexes with a coordination number of only three, which differ greatly from iron complexes with octahedral (six-coordinate) geometries with respect to their magnetism, electronic structure, preference for ligands, and reactivity. Three-coordinate complexes with a trigonal-planar geometry are accessible using bulky, anionic, bidentate ligands (beta-diketiminates) that steer a monodentate ligand into the plane of their two nitrogen donors. This strategy has led to a variety of three-coordinate iron complexes in which iron is in the +1, +2, and +3 oxidation states. Systematic studies on the electronic structures of these complexes have been useful in interpreting their properties. The iron ions are generally high spin, with singly occupied orbitals available for pi interactions with ligands. Trends in sigma-bonding show that iron(II) complexes favor electronegative ligands (O, N donors) over electropositive ligands (hydride). The combination of electrostatic sigma-bonding and the availability of pi-interactions stabilizes iron(II) fluoride and oxo complexes. The same factors destabilize iron(II) hydride complexes, which are reactive enough to add the hydrogen atom to unsaturated organic molecules and to take part in radical reactions. Iron(I) complexes use strong pi-backbonding to transfer charge from iron into coordinated alkynes and N 2, whereas iron(III) accepts charge from a pi-donating imido ligand. Though the imidoiron(III) complex is stabilized by pi-bonding in the trigonal-planar geometry, addition of pyridine as a fourth donor weakens the pi-bonding, which enables abstraction of H atoms from hydrocarbons. The unusual bonding and reactivity patterns of three-coordinate iron compounds may lead to new catalysts for oxidation and reduction reactions and may be used by nature in transient intermediates of nitrogenase enzymes.
Figures
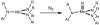
References
-
- Bradley DC, Chisholm MH. Transition-metal dialkylamides and disilylamides. Acc. Chem. Res. 1976;9:273–280.
-
- Cummins CC. Three-coordinate complexes of ‘hard’ ligands. Prog. Inorg. Chem. 1998;47:685–836.
-
- LaPointe RE, Wolczanski PT, Mitchell JF. Carbon monoxide cleavage by (silox)3Ta. J. Am. Chem. Soc. 1986;108:6382–6384.
-
- Cummins CC, Baxter SM, Wolczanski PT. Methane and benzene activation via transient (tert-(tBu3SiNH)2Zr=NSitBu3. J. Am. Chem. Soc. 1988;110:8731–8733.
- Schaller CP, Wolczanski PT. Methane vs benzene activation via transient tantalum amido-imido complex tBu3SiNHTa(=NSitBu3)2. Inorg. Chem. 1993;32:131–144.
-
- Laplaza CE, Cummins CC. Dinitrogen cleavage by a three-coordinate molybdenum(III) complex. Science. 1995;268:861–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

