The membrane-proximal intermolecular disulfide bonds in glycoprotein Ib influence receptor binding to von Willebrand factor
- PMID: 18647229
- PMCID: PMC2671080
- DOI: 10.1111/j.1538-7836.2008.03088.x
The membrane-proximal intermolecular disulfide bonds in glycoprotein Ib influence receptor binding to von Willebrand factor
Abstract
Background: In the platelet glycoprotein (GP)Ib-IX complex, the binding site for its ligand von Willebrand factor (VWF) is restricted to the N-terminal domain of the GPIbalpha subunit. How the other subunits in the complex, GPIbbeta and GPIX, regulate the GPIbalpha-VWF interaction is not clear.
Objectives and methods: As GPIbalpha connects with two GPIbbeta subunits via disulfide bonds, we tested whether these intersubunit covalent links were important to the proper VWF-binding activity of the GPIb-IX complex by characterizing the structure and VWF-binding activity of a mutant GPIb-IX complex that lacked the GPIbalpha-GPIbbeta disulfide bonds.
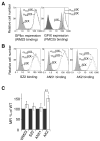
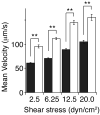
Results: Mutating both Cys484 and Cys485 of GPIbalpha to serine prevents GPIbalpha from forming covalent disulfide bonds with GPIbbeta, while maintaining the integrity of the complex in the membrane. The mutations cause two GPIbbeta subunits to form a disulfide bond between themselves. As compared to Chinese hamster ovary (CHO) cells stably expressing the wild-type GPIb-IX complex at a comparable level, CHO cells stably expressing the mutant GPIb-IX complex bind to significantly less soluble VWF in the presence of ristocetin and roll on the immobilized VWF under flow at a higher velocity.
Conclusions: The disulfide bonds between GPIbalpha and GPIbbeta are necessary for optimal GPIbalpha binding to VWF. The structural plasticity around the disulfide bonds may also help to shed light on the inside-out mechanism underlying GPIbbeta modulation of VWF binding.
Figures




Similar articles
-
The organizing principle of the platelet glycoprotein Ib-IX-V complex.J Thromb Haemost. 2013 Apr;11(4):605-14. doi: 10.1111/jth.12144. J Thromb Haemost. 2013. PMID: 23336709 Free PMC article. Review.
-
Identification of a novel 14-3-3zeta binding site within the cytoplasmic domain of platelet glycoprotein Ibalpha that plays a key role in regulating the von Willebrand factor binding function of glycoprotein Ib-IX.Circ Res. 2009 Dec 4;105(12):1177-85. doi: 10.1161/CIRCRESAHA.109.204669. Epub 2009 Oct 29. Circ Res. 2009. PMID: 19875727
-
Glycoprotein (GP) Ib-IX-transfected cells roll on a von Willebrand factor matrix under flow. Importance of the GPib/actin-binding protein (ABP-280) interaction in maintaining adhesion under high shear.J Biol Chem. 1999 Mar 5;274(10):6097-106. doi: 10.1074/jbc.274.10.6097. J Biol Chem. 1999. PMID: 10037692
-
Differential regulation of the platelet GPIb-IX complex by anti-GPIbβ antibodies.J Thromb Haemost. 2021 Aug;19(8):2044-2055. doi: 10.1111/jth.15359. Epub 2021 May 20. J Thromb Haemost. 2021. PMID: 33915031 Free PMC article.
-
Platelet physiology and thrombosis.Thromb Res. 2004;114(5-6):447-53. doi: 10.1016/j.thromres.2004.07.020. Thromb Res. 2004. PMID: 15507277 Review.
Cited by
-
The organizing principle of the platelet glycoprotein Ib-IX-V complex.J Thromb Haemost. 2013 Apr;11(4):605-14. doi: 10.1111/jth.12144. J Thromb Haemost. 2013. PMID: 23336709 Free PMC article. Review.
-
Unaccompanied mechanosensory domain mediates low expression of glycoprotein Ibα: implications for Bernard-Soulier syndrome.J Thromb Haemost. 2020 Feb;18(2):510-517. doi: 10.1111/jth.14684. Epub 2019 Dec 22. J Thromb Haemost. 2020. PMID: 31749281 Free PMC article.
-
Identifying key juxtamembrane interactions in cell membranes using AraC-based transcriptional reporter assay (AraTM).J Biol Chem. 2012 Sep 7;287(37):31515-26. doi: 10.1074/jbc.M112.396895. Epub 2012 Jul 22. J Biol Chem. 2012. PMID: 22822084 Free PMC article.
-
Transmembrane domains are critical to the interaction between platelet glycoprotein V and glycoprotein Ib-IX complex.J Thromb Haemost. 2012 Sep;10(9):1875-86. doi: 10.1111/j.1538-7836.2012.04841.x. J Thromb Haemost. 2012. PMID: 22759073 Free PMC article.
-
Transmembrane and trans-subunit regulation of ectodomain shedding of platelet glycoprotein Ibalpha.J Biol Chem. 2010 Oct 15;285(42):32096-104. doi: 10.1074/jbc.M110.111864. Epub 2010 Aug 17. J Biol Chem. 2010. PMID: 20716526 Free PMC article.
References
-
- Berndt MC, Shen Y, Dopheide SM, Gardiner EE, Andrews RK. The vascular biology of the glycoprotein Ib-IX-V complex. Thromb Haemost. 2001;86:178–88. - PubMed
-
- Cauwenberghs N, Vanhoorelbeke K, Vauterin S, Deckmyn H. Structural determinants within platelet glycoprotein Ibαinvolved in its binding to von Willebrand factor. Platelets. 2000;11:373–8. - PubMed
-
- Dong JF, Li CQ, Lopez JA. Tyrosine sulfation of the glycoprotein Ib-IX complex: identification of sulfated residues and effect on ligand binding. Biochemistry. 1994;33:13946–53. - PubMed
-
- Shen Y, Dong JF, Romo GM, Arceneaux W, Aprico A, Gardiner EE, Lopez JA, Berndt MC, Andrews RK. Functional analysis of the C-terminal flanking sequence of platelet glycoprotein Ibαusing canine-human chimeras. Blood. 2002;99:145–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

