Role of dichloroacetate in the treatment of genetic mitochondrial diseases
- PMID: 18647626
- PMCID: PMC3746325
- DOI: 10.1016/j.addr.2008.02.014
Role of dichloroacetate in the treatment of genetic mitochondrial diseases
Abstract
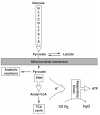
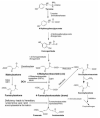
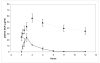
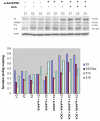
Dichloroacetate (DCA) is an investigational drug for the treatment of genetic mitochondrial diseases. Its primary site of action is the pyruvate dehydrogenase (PDH) complex, which it stimulates by altering its phosphorylation state and stability. DCA is metabolized by and inhibits the bifunctional zeta-1 family isoform of glutathione transferase/maleylacetoacetate isomerase. Polymorphic variants of this enzyme differ in their kinetic properties toward DCA, thereby influencing its biotransformation and toxicity, both of which are also influenced by subject age. Results from open label studies and controlled clinical trials suggest chronic oral DCA is generally well-tolerated by young children and may be particularly effective in patients with PDH deficiency. Recent in vitro data indicate that a combined DCA and gene therapy approach may also hold promise for the treatment of this devastating condition.
Figures




References
-
- Stacpoole PW. Review of the pharmacologic and therapeutic effects of diisopropylammonium dichloroacetate (DIPA) J Clin Pharmacol J New Drugs. 1969;9:282–291. - PubMed
-
- Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, Felitsyn NM, Gilmore RL, Greer M, Henderson GN, Hutson AD, Neiberger RE, O’Brien RG, Perkins LE, Quisling RG, Shroads AL, Shuster JJ, Silverstein JH, Theriaque DW, Valenstein E. A controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117:1519–1521. - PubMed
-
- Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, Millar WS, Hong X, Gooch CL, Mao X, Pascual JM, Hirano M, Stacpoole PW, DiMauro S, DeVivo DC. Dichloroacetate as a treatment for MELAS: A randomized, controlled clinical trial. Neurology. 2006;66:324–30. - PubMed
-
- Curry SH, Lorenz A, Chu PI, Limacher M, Stacpoole PW. Disposition and pharmacodynamics of dichloroacetate (DCA) and oxalate following oral DCA doses. Biopharm Drug Disp. 1991;12:375–390. - PubMed
-
- Ammini CV, Stacpoole PW. Biotransformation, Toxicology and Pharmacogenomics of Dichloroacetate. In: Gribble GW, editor. Natural Production of Organohalogen Compounds. Vol. 3/P in the series The Handbook of Environmental Chemistry. Springer-Verlag; Berlin:New York: 2003. pp. 215–234.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

