Complementary vasoactivity and matrix remodelling in arterial adaptations to altered flow and pressure
- PMID: 18647735
- PMCID: PMC2659584
- DOI: 10.1098/rsif.2008.0254
Complementary vasoactivity and matrix remodelling in arterial adaptations to altered flow and pressure
Abstract
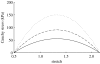
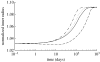
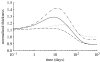
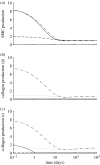
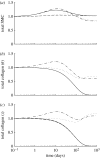
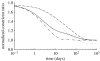
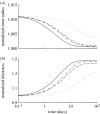
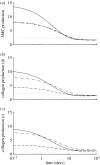
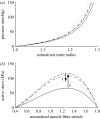
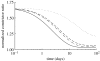
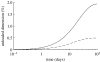
Arteries exhibit a remarkable ability to adapt to sustained alterations in biomechanical loading, probably via mechanisms that are similarly involved in many arterial pathologies and responses to treatment. Of particular note, diverse data suggest that cell and matrix turnover within vasoaltered states enables arteries to adapt to sustained changes in blood flow and pressure. The goal herein is to show explicitly how altered smooth muscle contractility and matrix growth and remodelling work together to adapt the geometry, structure, stiffness and function of a representative basilar artery. Towards this end, we employ a continuum theory of constrained mixtures to model evolving changes in the wall, which depend on both wall shear stress-induced changes in vasoactive molecules (which alter smooth muscle proliferation and synthesis of matrix) and intramural stress-induced changes in growth factors (which alter cell and matrix turnover). Simulations show, for example, that such considerations help explain the different rates of experimentally observed adaptations to increased versus decreased flows as well as differences in rates of change in response to increased flows or pressures.
Figures





References
-
- Brownlee R.D., Langille B.L. Arterial adaptations to altered blood flow. Can. J. Physiol. Pharmacol. 1991;69:978–983. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
