Mechanism for alternating access in neurotransmitter transporters
- PMID: 18647834
- PMCID: PMC2480614
- DOI: 10.1073/pnas.0804659105
Mechanism for alternating access in neurotransmitter transporters
Abstract
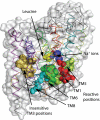
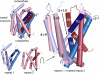
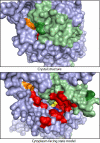
Crystal structures of LeuT, a bacterial homologue of mammalian neurotransmitter transporters, show a molecule of bound substrate that is essentially exposed to the extracellular space but occluded from the cytoplasm. Thus, there must exist an alternate conformation for LeuT in which the substrate is accessible to the cytoplasm and a corresponding mechanism that switches accessibility from one side of the membrane to the other. Here, we identify the cytoplasmic accessibility pathway of the alternate conformation in a mammalian serotonin transporter (SERT) (a member of the same transporter family as LeuT). We also propose a model for the cytoplasmic-facing state that exploits the internal pseudosymmetry observed in the crystal structure. LeuT contains two structurally similar repeats (TMs1-5 and TMs 6-10) that are inverted with respect to the plane of the membrane. The conformational differences between them result in the formation of the extracellular pathway. Our model for the cytoplasm-facing state exchanges the conformations of the two repeats and thus exposes the substrate and ion-binding sites to the cytoplasm. The conformational change that connects the two states primarily involves the tilting of a 4-helix bundle composed of transmembrane helices 1, 2, 6, and 7. Switching the tilt angle of this bundle is essentially equivalent to switching the conformation of the two repeats. Extensive mutagenesis of SERT and accessibility measurements, using cysteine reagents, are accommodated by our model. These observations may be of relevance to other transporter families, many of which contain internal inverted repeats.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. - PubMed
-
- Olesen C, et al. The structural basis of calcium transport by the calcium pump. Nature. 2007;450:1036–1042. - PubMed
-
- Hirai T, et al. Three-dimensional structure of a bacterial oxalate transporter. Nat Struct Biol. 2002;9:597–600. 2002. - PubMed
-
- Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

