Analysis of glycosyltransferase expression in metastatic prostate cancer cells capable of rolling activity on microvascular endothelial (E)-selectin
- PMID: 18647941
- PMCID: PMC2574550
- DOI: 10.1093/glycob/cwn070
Analysis of glycosyltransferase expression in metastatic prostate cancer cells capable of rolling activity on microvascular endothelial (E)-selectin
Abstract
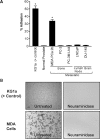
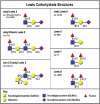
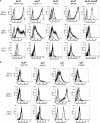
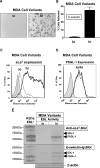
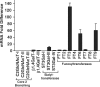
Prostate cancer (PCa) cell tethering and rolling on microvascular endothelium has been proposed to promote the extravasation of PCa cells. We have shown that these adhesive events are mediated through binding interactions between endothelial (E)-selectin and Lewis carbohydrates on PCa cells. Prior data indicate that E-selectin-mediated rolling of bone-metastatic PCa MDA PCa 2b (MDA) cells is dependent on sialyl Lewis X (sLe(X))-bearing glycoproteins. To explore the molecular basis of sLe(X) synthesis and E-selectin ligand (ESL) activity on PCa cells, we compared and contrasted the expression level of glycosyltransferases, characteristically involved in sLe(X) and ESL synthesis, in ESL(+) MDA cells among other ESL(-) metastatic PCa cell lines. We also created and examined ESL(hi) and ESL(lo) variants of MDA cells to provide a direct comparison of the glycosyltransferase expression level. We found that normal prostate tissue and all metastatic PCa cell lines expressed glycosyltransferases required for sialo-lactosamine synthesis, including N-acetylglucosaminyl-, galactosyl-, and sialyltransferases. However, compared with expression in normal prostate tissue, ESL(+) MDA cells expressed a 31- and 10-fold higher level of alpha1,3 fucosyltransferases (FT) 3 and 6, respectively. Moreover, FT3 and FT6 were expressed at 2- to 354-fold lower levels in ESL(-) PCa cell lines. Consistent with these findings, ESL(hi) MDA cells expressed a 131- and 51-fold higher level of FT3 and FT6, respectively, compared with expression in ESL(lo) MDA cells. We also noted that alpha1,3 FT7 was expressed at a 5-fold greater level in ESL(hi) MDA cells. Furthermore, ESL(lo) MDA cells did not display sLe(X) on glycoproteins capable of bearing sLe(X), notably P-selectin glycoprotein ligand-1. These results implicate the importance of alpha1,3 FT3, FT6, and/or FT7 in sLe(X) and ESL synthesis on metastatic PCa cells.
Figures

References
-
- Aubert M, Panicot-Dubois L, Crotte C, Sbarra V, Lombardo D, Sadoulet MO, Mas E. Peritoneal colonization by human pancreatic cancer cells is inhibited by antisense FUT3 sequence. Int J Cancer. 2000;88:558–565. - PubMed
-
- Benharroch D, Dima E, Levy A, Ohana-Malka O, Ariad S, Prinsloo I, Mejirovsky E, Sacks M, Gopas J. Differential expression of sialyl and non-sialyl-CD15 antigens on Hodgkin-Reed-Sternberg cells: Significance in Hodgkin's disease. Leuk Lymphoma. 2000;39:185–194. - PubMed
-
- Benjamin R. Neurologic complications of prostate cancer. Am Fam Physician. 2002;65:1834–1840. - PubMed
-
- Biol-N’garagba MC, Niepceron E, Mathian B, Louisot P. Glucocorticoid-induced maturation of glycoprotein galactosylation and fucosylation processes in the rat small intestine. J Steroid Biochem Mol Biol. 2003;84:411–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

