Pathologic modifications of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated squirrel monkeys
- PMID: 18648323
- PMCID: PMC2745435
- DOI: 10.1097/NEN.0b013e318180f0bd
Pathologic modifications of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated squirrel monkeys
Abstract
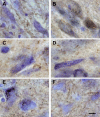
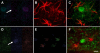
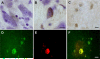
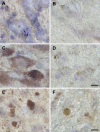
alpha-Synuclein expression is increased in dopaminergic neurons challenged by toxic insults. Here, we assessed whether this upregulation is accompanied by pathologic accumulation of alpha-synuclein and protein modifications (i.e. nitration, phosphorylation, and aggregation) that are typically observed in Parkinson disease and in other synucleinopathies. A single injection of the neurotoxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to squirrel monkeys caused a buildup of alpha-synuclein but not of beta-synuclein or synaptophysin within nigral dopaminergic cell bodies. Immunohistochemistry and immunoelectron microscopy also revealed large numbers of dystrophic axons labeled with alpha-synuclein. Antibodies that recognize nitrated and phosphorylated (at serine 129) alpha-synuclein stained neuronal cell bodies and dystrophic axons in the midbrain of MPTP-treated animals. After toxicant exposure, alpha-synuclein deposition occurred at the level of neuronal axons in which amorphous protein aggregates were observed by immunoelectron microscopy. In a subset of these axons, immunoreactivity for alpha-synuclein was still evident after tissue digestion with proteinase K, further indicating the accumulation of insoluble protein. These data indicate that toxic injury can induce alpha-synuclein modifications that have been implicated in the pathogenesis of human synucleinopathies. The findings are also consistent with a pattern of evolution of alpha-synuclein pathology that may begin with the accumulation and aggregation of the protein within damaged axons.
Figures



Similar articles
-
Alpha-synuclein expression in the substantia nigra of MPTP-lesioned non-human primates.Neurobiol Dis. 2005 Dec;20(3):898-906. doi: 10.1016/j.nbd.2005.05.028. Epub 2005 Jul 11. Neurobiol Dis. 2005. PMID: 16006134
-
Overexpression of Parkinson's disease-associated alpha-synucleinA53T by recombinant adeno-associated virus in mice does not increase the vulnerability of dopaminergic neurons to MPTP.J Neurobiol. 2002 Oct;53(1):1-10. doi: 10.1002/neu.10094. J Neurobiol. 2002. PMID: 12360578
-
Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP.J Neurochem. 2000 Feb;74(2):721-9. doi: 10.1046/j.1471-4159.2000.740721.x. J Neurochem. 2000. PMID: 10646524
-
What have we learnt from CDNA microarray gene expression studies about the role of iron in MPTP induced neurodegeneration and Parkinson's disease?J Neural Transm Suppl. 2003;(65):73-88. doi: 10.1007/978-3-7091-0643-3_5. J Neural Transm Suppl. 2003. PMID: 12946050 Review.
-
alpha-Synuclein- and MPTP-generated rodent models of Parkinson's disease and the study of extracellular striatal dopamine dynamics: a microdialysis approach.CNS Neurol Disord Drug Targets. 2010 Aug;9(4):482-90. doi: 10.2174/187152710791556177. CNS Neurol Disord Drug Targets. 2010. PMID: 20522009 Review.
Cited by
-
Lysosomal degradation of alpha-synuclein in vivo.J Biol Chem. 2010 Apr 30;285(18):13621-9. doi: 10.1074/jbc.M109.074617. Epub 2010 Mar 3. J Biol Chem. 2010. PMID: 20200163 Free PMC article.
-
Essential Oils as a Potential Neuroprotective Remedy for Age-Related Neurodegenerative Diseases: A Review.Molecules. 2021 Feb 19;26(4):1107. doi: 10.3390/molecules26041107. Molecules. 2021. PMID: 33669787 Free PMC article. Review.
-
Involvement of the Fc gamma receptor in a chronic N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of dopaminergic loss.J Biol Chem. 2011 Aug 19;286(33):28783-28793. doi: 10.1074/jbc.M111.244830. Epub 2011 Jun 21. J Biol Chem. 2011. PMID: 21693708 Free PMC article.
-
Animal models of Parkinson's disease: limits and relevance to neuroprotection studies.Mov Disord. 2013 Jan;28(1):61-70. doi: 10.1002/mds.25108. Epub 2012 Jul 2. Mov Disord. 2013. PMID: 22753348 Free PMC article. Review.
-
Selective vulnerability in α-synucleinopathies.Acta Neuropathol. 2019 Nov;138(5):681-704. doi: 10.1007/s00401-019-02010-2. Epub 2019 Apr 20. Acta Neuropathol. 2019. PMID: 31006067 Free PMC article. Review.
References
-
- Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–47. - PubMed
-
- Kruger R, Kuhn W, Muller T, et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. - PubMed
-
- Zarranz JJ, Alegre J, Gomez-Esteban JC, et al. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–73. - PubMed
-
- Tu PH, Galvin JE, Baba M, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann Neurol. 1998;44:415–22. - PubMed

