Relationships between expression of apolipoprotein E and beta-amyloid precursor protein are altered in proximity to Alzheimer beta-amyloid plaques: potential explanations from cell culture studies
- PMID: 18648325
- PMCID: PMC3334532
- DOI: 10.1097/NEN.0b013e318180ec47
Relationships between expression of apolipoprotein E and beta-amyloid precursor protein are altered in proximity to Alzheimer beta-amyloid plaques: potential explanations from cell culture studies
Abstract
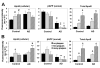
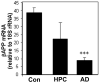
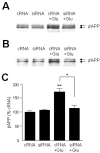
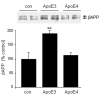
Theories regarding the initiation and progression of Alzheimer disease (AD) often consider potential roles played by elevations of beta-amyloid precursor protein (betaAPP). Because it is the source of amyloid beta-peptide, betaAPP may simply contribute more pathogenic stimulus when elevated; some analyses have, however, reported a decline in betaAPP in AD. We found a progressive increase in neuronal betaAPP expression with increasing age in the brains of nondemented individuals, whereas in AD patient samples, betaAPP antigenicity decreased in neuronal somata in a manner that correlated with accumulation of mature amyloid beta-peptide plaques. In contrast, apolipoprotein E (ApoE) expression correlated with accumulation of plaques, and even greater amounts of ApoE were detected in plaques. Induction of betaAPP by glutamate in neuronal cell cultures was found to depend upon ApoE levels or activity. Thus, elevations in expression of ApoE and betaAPP by cellular stresses are likely normally linked in vivo, and uncoupling of this link, or other pathologic events in AD initiation, may leave neurons with diminished betaAPP expression, which might in turn reduce their resistance to stressors.
Figures




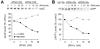

References
-
- Dawson GR, Seabrook GR, Zheng H, et al. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. - PubMed
-
- Masliah E, Westland CE, Rockenstein EM, et al. Amyloid precursor proteins protect neurons of transgenic mice against acute and chronic excitotoxic injuries in vivo. Neuroscience. 1997;78:135–46. - PubMed
-
- Prasher VP, Farrer MJ, Kessling AM, et al. Molecular mapping of Alzheimer-type dementia in Down's syndrome. Ann Neurol. 1998;43:380–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

