Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy
- PMID: 18648503
- PMCID: PMC2447178
- DOI: 10.1371/journal.pone.0002748
Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy
Abstract
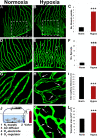
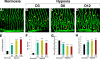
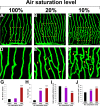
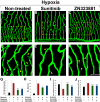
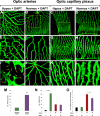
Mechanistic understanding and defining novel therapeutic targets of diabetic retinopathy and age-related macular degeneration (AMD) have been hampered by a lack of appropriate adult animal models. Here we describe a simple and highly reproducible adult fli-EGFP transgenic zebrafish model to study retinal angiogenesis. The retinal vasculature in the adult zebrafish is highly organized and hypoxia-induced neovascularization occurs in a predictable area of capillary plexuses. New retinal vessels and vascular sprouts can be accurately measured and quantified. Orally active anti-VEGF agents including sunitinib and ZM323881 effectively block hypoxia-induced retinal neovascularization. Intriguingly, blockage of the Notch signaling pathway by the inhibitor DAPT under hypoxia, results in a high density of arterial sprouting in all optical arteries. The Notch suppression-induced arterial sprouting is dependent on tissue hypoxia. However, in the presence of DAPT substantial endothelial tip cell formation was detected only in optic capillary plexuses under normoxia. These findings suggest that hypoxia shifts the vascular targets of Notch inhibitors. Our findings for the first time show a clinically relevant retinal angiogenesis model in adult zebrafish, which might serve as a platform for studying mechanisms of retinal angiogenesis, for defining novel therapeutic targets, and for screening of novel antiangiogenic drugs.
Conflict of interest statement
Figures
Similar articles
-
Hypoxia-induced retinal neovascularization in zebrafish embryos: a potential model of retinopathy of prematurity.PLoS One. 2015 May 15;10(5):e0126750. doi: 10.1371/journal.pone.0126750. eCollection 2015. PLoS One. 2015. PMID: 25978439 Free PMC article.
-
Regulation of ocular angiogenesis by Notch signaling: implications in neovascular age-related macular degeneration.Invest Ophthalmol Vis Sci. 2011 May 2;52(6):2868-78. doi: 10.1167/iovs.10-6608. Invest Ophthalmol Vis Sci. 2011. PMID: 21228388 Free PMC article.
-
Hypoxia-induced retinopathy model in adult zebrafish.Nat Protoc. 2010 Dec;5(12):1903-10. doi: 10.1038/nprot.2010.149. Epub 2010 Nov 4. Nat Protoc. 2010. PMID: 21127484
-
Zebrafish (Danio rerio) embryo as a platform for the identification of novel angiogenesis inhibitors of retinal vascular diseases.Biochim Biophys Acta. 2016 Jul;1862(7):1291-6. doi: 10.1016/j.bbadis.2016.04.009. Epub 2016 Apr 13. Biochim Biophys Acta. 2016. PMID: 27085972 Review.
-
Molecular pathogenesis of retinal and choroidal vascular diseases.Prog Retin Eye Res. 2015 Nov;49:67-81. doi: 10.1016/j.preteyeres.2015.06.002. Epub 2015 Jun 23. Prog Retin Eye Res. 2015. PMID: 26113211 Free PMC article. Review.
Cited by
-
Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model.Proc Natl Acad Sci U S A. 2009 Nov 17;106(46):19485-90. doi: 10.1073/pnas.0909228106. Epub 2009 Nov 3. Proc Natl Acad Sci U S A. 2009. PMID: 19887629 Free PMC article.
-
Erianin Controls Collagen-Mediated Retinal Angiogenesis via the RhoA/ROCK1 Signaling Pathway Induced by the alpha2/beta1 Integrin-Collagen Interaction.Invest Ophthalmol Vis Sci. 2022 Jan 3;63(1):27. doi: 10.1167/iovs.63.1.27. Invest Ophthalmol Vis Sci. 2022. PMID: 35060996 Free PMC article.
-
Animal models of diabetic retinopathy.Ann Transl Med. 2021 Aug;9(15):1272. doi: 10.21037/atm-20-6737. Ann Transl Med. 2021. PMID: 34532409 Free PMC article. Review.
-
Pentose Pathway Activation Is Superior to Increased Glycolysis for Therapeutic Angiogenesis in Peripheral Arterial Disease.J Am Heart Assoc. 2023 Apr 4;12(7):e027986. doi: 10.1161/JAHA.122.027986. Epub 2023 Mar 28. J Am Heart Assoc. 2023. PMID: 36974760 Free PMC article.
-
Chronic whole-body hypoxia induces intussusceptive angiogenesis and microvascular remodeling in the mouse retina.Microvasc Res. 2010 Mar;79(2):93-101. doi: 10.1016/j.mvr.2010.01.006. Epub 2010 Jan 18. Microvasc Res. 2010. PMID: 20080108 Free PMC article.
References
-
- Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353:839–841. - PubMed
-
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. - PubMed
-
- Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–992. - PubMed
-
- Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. - PubMed
-
- Gardner TW, Antonetti DA. A prize catch for diabetic retinopathy. Nat Med. 2007;13:131–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

