Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice
- PMID: 18648532
- PMCID: PMC2481277
- DOI: 10.1371/journal.pone.0002740
Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice
Abstract
Background: Innate neuroimmune dysfunction is a pathobiological feature of amyotrophic lateral sclerosis (ALS). However, links, if any, between disease and adaptive immunity are poorly understood. Thus, the role of T cell immunity in disease was investigated in human G93A superoxide dismutase 1 (SOD1) transgenic (Tg) mice and subsequently in ALS patients.
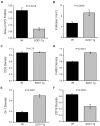
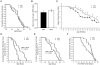
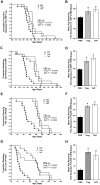
Methods and findings: Quantitative and qualitative immune deficits in lymphoid cell and T cell function were seen in G93A-SOD1 Tg mice. Spleens of Tg animals showed reductions in size, weight, lymphocyte numbers, and morphological deficits at terminal stages of disease compared to their wild-type (Wt) littermates. Spleen sizes and weights of pre-symptomatic Tg mice were unchanged, but deficits were readily seen in T cell proliferation coincident with increased annexin-V associated apoptosis and necrosis of lymphocytes. These lymphoid deficits paralleled failure of Copolymer-1 (COP-1) immunization to affect longevity. In addition, among CD4(+) T cells in ALS patients, levels of CD45RA(+) (naïve) T cells were diminished, while CD45RO(+) (memory) T cells were increased compared to age-matched caregivers. In attempts to correct mutant SOD1 associated immune deficits, we reconstituted SOD1 Tg mice with unfractionated naïve lymphocytes or anti-CD3 activated CD4(+)CD25(+) T regulatory cells (Treg) or CD4(+)CD25(-) T effector cells (Teff) from Wt donor mice. While naive lymphocytes failed to enhance survival, both polyclonal-activated Treg and Teff subsets delayed loss of motor function and extended survival; however, only Treg delayed neurological symptom onset, whereas Teff increased latency between disease onset and entry into late stage.
Conclusions: A profound and progressive immunodeficiency is operative in G93A-SOD1 mice and is linked to T cell dysfunction and the failure to elicit COP-1 neuroprotective immune responses. In preliminary studies T cell deficits were also observed in human ALS. These findings, taken together, suggest caution in ascribing vaccination outcomes when these animal models of human ALS are used for study. Nonetheless, the abilities to improve neurological function and life expectancy in G93A-SOD1 Tg mice by reconstitution with activated T cells do provide opportunities for therapeutic intervention.
Conflict of interest statement
Figures






References
-
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. - PubMed
-
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. - PubMed
-
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. - PubMed
-
- Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS11766/NS/NINDS NIH HHS/United States
- R01 AG021617/AG/NIA NIH HHS/United States
- P50 NS038370/NS/NINDS NIH HHS/United States
- AG021617/AG/NIA NIH HHS/United States
- R21 NS049264/NS/NINDS NIH HHS/United States
- R21 ES013177/ES/NIEHS NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- NS007488/NS/NINDS NIH HHS/United States
- R01 NS042269/NS/NINDS NIH HHS/United States
- NS42269/NS/NINDS NIH HHS/United States
- NS36136/NS/NINDS NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- NS38370/NS/NINDS NIH HHS/United States
- ES013177/ES/NIEHS NIH HHS/United States
- MH64570/MH/NIMH NIH HHS/United States
- NS049264/NS/NINDS NIH HHS/United States
- NS43985/NS/NINDS NIH HHS/United States
- P01 NS011766/NS/NINDS NIH HHS/United States
- T32 NS007488/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

