Cell proliferation, reactive oxygen and cellular glutathione
- PMID: 18648617
- PMCID: PMC2475947
- DOI: 10.2203/dose-response.003.03.010
Cell proliferation, reactive oxygen and cellular glutathione
Abstract
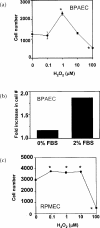
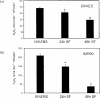
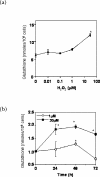
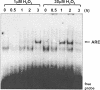
A variety of cellular activities, including metabolism, growth, and death, are regulated and modulated by the redox status of the environment. A biphasic effect has been demonstrated on cellular proliferation with reactive oxygen species (ROS)-especially hydrogen peroxide and superoxide-in which low levels (usually submicromolar concentrations) induce growth but higher concentrations (usually >10-30 micromolar) induce apoptosis or necrosis. This phenomenon has been demonstrated for primary, immortalized and transformed cell types. However, the mechanism of the proliferative response to low levels of ROS is not well understood. Much of the work examining the signal transduction by ROS, including H(2)O(2), has been performed using doses in the lethal range. Although use of higher ROS doses have allowed the identification of important signal transduction pathways, these pathways may be activated by cells only in association with ROS-induced apoptosis and necrosis, and may not utilize the same pathways activated by lower doses of ROS associated with increased cell growth. Recent data has shown that low levels of exogenous H(2)O(2) up-regulate intracellular glutathione and activate the DNA binding activity toward antioxidant response element. The modulation of the cellular redox environment, through the regulation of cellular glutathione levels, may be a part of the hormetic effect shown by ROS on cell growth.
Keywords: MAPK; antioxidant response element; biphasic response; oxidative stress; signal transduction.
Figures


References
-
- Babior B.M. The NADPH oxidase of endothelial cells. IUBMB Life. 2000;50:267–9. - PubMed
-
- Bayraktutan U., Blayney L., Shah A.M. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1903–11. - PubMed
-
- Boonstra J., Post J.A. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

