Deleted in liver cancer 1 (DLC1) negatively regulates Rho/ROCK/MLC pathway in hepatocellular carcinoma
- PMID: 18648664
- PMCID: PMC2464714
- DOI: 10.1371/journal.pone.0002779
Deleted in liver cancer 1 (DLC1) negatively regulates Rho/ROCK/MLC pathway in hepatocellular carcinoma
Abstract
Aims: Deleted in liver cancer 1 (DLC1), a member of RhoGTPase activating protein (GAP) family, is known to have suppressive activities in tumorigenicity and cancer metastasis. However, the underlying molecular mechanisms of how DLC1 suppresses cell motility have not been fully elucidated. Rho-kinase (ROCK) is an immediate down-stream effector of RhoA in mediating cellular cytoskeletal events and cell motility. In the present study, we aimed to investigate the effects of DLC1 on Rho/ROCK signaling pathway in hepatocellular carcinoma (HCC).
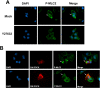
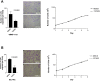
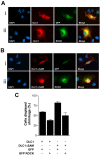
Methodology/principal findings: We demonstrated that DLC1 negatively regulated ROCK-dependent actomyosin contractility. From immunofluorescence study, we found that ectopic expression of DLC1 abrogated Rho/ROCK-mediated cytoskeletal reorganization including formation of stress fibers and focal adhesions. It also downregulated cortical phosphorylation of myosin light chain 2 (MLC2). These inhibitory events by DLC1 were RhoGAP-dependent, as RhoGAP-deficient mutant of DLC1 (DLC1 K714E) abolished these inhibitory events. In addition, from western study, DLC1 inhibited ROCK-related myosin light chain phosphatase targeting unit 1 (MYPT1) phosphorylation at Threonine 853. By examining cell morphology under microscope, we found that ectopic expression of dominant-active ROCK released cells from DLC1-induced cytoskeletal collapse and cell shrinkage.
Conclusion: Our data suggest that DLC1 negatively regulates Rho/ROCK/MLC2. This implicates a ROCK-mediated pathway of DLC1 in suppressing metastasis of HCC cells and enriches our understanding in the molecular mechanisms involved in the progression of hepatocellular carcinoma.
Conflict of interest statement
Figures





References
-
- Yuan BZ, Miller MJ, Keck CL, Zimonjic DB, Thorgeirsson SS, et al. Cloning, characterization, and chromosomal localization of a gene frequently deleted in human liver cancer (DLC-1) homologous to rat RhoGAP. Cancer Res. 1998;58:2196–2199. - PubMed
-
- Wong CM, Yam JW, Ching YP, Yau TO, Leung TH, et al. Rho GTPase-activating protein deleted in liver cancer suppresses cell proliferation and invasion in hepatocellular carcinoma. Cancer Res. 2005;65:8861–8868. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

