Lens fiber connexin turnover and caspase-3-mediated cleavage are regulated alternately by phosphorylation
- PMID: 18649174
- PMCID: PMC2719281
- DOI: 10.1080/15419060802253663
Lens fiber connexin turnover and caspase-3-mediated cleavage are regulated alternately by phosphorylation
Abstract
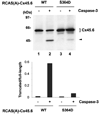
Lens connexins are phosphorylated in vivo; however, the function and regulation of the phosphorylation remain largely unknown. We have previously identified an in vivo phosphorylation site, Ser(364), at the COOH terminus of lens connexin (Cx) Cx45.6 and phosphorylation appears to regulate connexin protein turnover. To assess the specific mechanism of Ser(364) phosphorylation in Cx45.6, exogenous wild type and Ser(364) mutant Cx45.6 were expressed in primary lens cultures through retroviral infection. Cx45.6 turnover was attenuated primarily by proteasomal inhibitors and to a lesser extent by lysosomal inhibitors. Furthermore, the level of Cx45.6 protein in ubiquitin co-expressed cells was significantly reduced as compared to the cells expressing Cx45.6 alone. Moreover, overexpression of ubiquitin led to a more significant decrease in wild type Cx45.6 than Cx45.6(S364A), a mutant deficient of phosphorylation site at Ser(364), although we did not detect any difference in the levels of ubiquitination between wild type and mutant Cx45.6. Interestingly, the mutant mimicking constitutive phosphorylation, Cx45.6(S364D), partially prevented the cleavage of Cx45.6 by caspase-3. Together, our data suggest that phosphorylation of Cx45.6 at Ser(364) appears to stimulate Cx45.6 turnover primarily through proteasome pathway and this phosphorylation inhibits the cleavage of Cx45.6 by caspase-3. These findings provide further insights into regulatory mechanism of the specific phosphorylation of connexins in the lens.
Figures



Similar articles
-
Gap junctions or hemichannel-dependent and independent roles of connexins in cataractogenesis and lens development.Curr Mol Med. 2010 Dec;10(9):851-63. doi: 10.2174/156652410793937750. Curr Mol Med. 2010. PMID: 21091421 Free PMC article. Review.
-
The development-associated cleavage of lens connexin 45.6 by caspase-3-like protease is regulated by casein kinase II-mediated phosphorylation.J Biol Chem. 2001 Sep 14;276(37):34567-72. doi: 10.1074/jbc.M106073200. Epub 2001 Jul 11. J Biol Chem. 2001. PMID: 11448971
-
Casein kinase II phosphorylates lens connexin 45.6 and is involved in its degradation.J Biol Chem. 2000 Mar 10;275(10):6850-6. doi: 10.1074/jbc.275.10.6850. J Biol Chem. 2000. PMID: 10702244
-
Regulation of lens connexin 45.6 by apoptotic protease, caspase-3.Cell Commun Adhes. 2001;8(4-6):373-6. doi: 10.3109/15419060109080756. Cell Commun Adhes. 2001. PMID: 12064621
-
Focus on lens connexins.BMC Cell Biol. 2017 Jan 17;18(Suppl 1):6. doi: 10.1186/s12860-016-0116-6. BMC Cell Biol. 2017. PMID: 28124626 Free PMC article. Review.
Cited by
-
Degradation of connexins and gap junctions.FEBS Lett. 2014 Apr 17;588(8):1221-9. doi: 10.1016/j.febslet.2014.01.031. Epub 2014 Jan 30. FEBS Lett. 2014. PMID: 24486527 Free PMC article. Review.
-
Connexin Mutants Compromise the Lens Circulation and Cause Cataracts through Biomineralization.Int J Mol Sci. 2020 Aug 13;21(16):5822. doi: 10.3390/ijms21165822. Int J Mol Sci. 2020. PMID: 32823750 Free PMC article. Review.
-
Posttranslational modifications in connexins and pannexins.J Membr Biol. 2012 Jun;245(5-6):319-32. doi: 10.1007/s00232-012-9453-3. Epub 2012 Jun 28. J Membr Biol. 2012. PMID: 22739962 Free PMC article. Review.
-
Gap junctions or hemichannel-dependent and independent roles of connexins in cataractogenesis and lens development.Curr Mol Med. 2010 Dec;10(9):851-63. doi: 10.2174/156652410793937750. Curr Mol Med. 2010. PMID: 21091421 Free PMC article. Review.
-
Connexin Controls Cell-Cycle Exit and Cell Differentiation by Directly Promoting Cytosolic Localization and Degradation of E3 Ligase Skp2.Dev Cell. 2015 Nov 23;35(4):483-96. doi: 10.1016/j.devcel.2015.10.014. Epub 2015 Nov 12. Dev Cell. 2015. PMID: 26585299 Free PMC article.
References
-
- Aviel S, Winberg G, Massucci M, Ciechanover A. Degradation of the Epstein-Barr latent membrane protein 1 (LMP) by the ubiquitin-proteasome pathway-targeting via ubiquitination of the N-terminal residue. J Biol Chem. 2000;275:23491–23499. - PubMed
-
- Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. - PubMed
-
- Bassnett S, Duncan G. Direct measurement of pH in the rat lens by ion-sensitive microelectrodes. Exp Eye Res. 1985;40:585–590. - PubMed
-
- Berthoud VM, Bassnett S, Beyer EC. Cultured chicken embryo lens cells resemble differentiating fiber cells in vivo and contain two kinetic pools of connexin56. Exp Eye Res. 1999;68:475–484. - PubMed
-
- Cheng HL, Louis CF. Endogenous casein kinase I catalyzes the phosphorylation of the lens fiber cell connexin49. Eur J Biochem. 1999;263:276–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
